| Pages:
1
2 |
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
Dehydration of 3-Phenyl-1-propanol
I've been searching high and low and haven't found any cheap methods for that particular dehydration, i would expect something like heating with
Na/KHSO4 furnishing Allyl- or Propenylbenzene.
So far i have found:
- Halogenation, displacement with trimethylamine and elimination of trimethylammonium halide.
- Treatment with SOCl2
- Tosylation with subsequent elimination,
all of which are far out of my range. This is just for fun 
I've got some leftover 3-Phenyl-1-propanol which i dont have any use for.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
That stuff, C6H5-CH2-CH2-CH2OH,
looks like it could be used as an intermediate in making methamphetamine and its derivatives, so at least we know that you are not a druggie, if you
say you have no use for it!
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
JohnWW: From what I can see the converse is correct, going directly from 3-phenylpropan-1-ol would not exactly be as easy as going via allylbenzene...
However, as the question was posed in a scientific manner, and with no reference to such use, I'll see what I can shine on it.
Crossfire comes up with very little other than SOCl2, Pyridine and subsequent pyrolysis of the sulfite ester.
However, looking for other reactions where your material is the substrate, I found a couple of reactions that may interest you. Both are
halogenations, and when I searched for the halide=>alkene, very little come up. I suspect the standard basic treatment (t-BuOK or KOH for example)
would yield the product you want. Find the papers attached.
Attachment: HCl, NaI.pdf (779kB)
This file has been downloaded 1214 times
Attachment: Al(HSO4)3 + KI.pdf (62kB)
This file has been downloaded 1504 times
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Wouldn't the standard dehydration reaction with phosphoric acid work? Or does it need to be a secondary alcohol.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
I recall a JACS article that achieved dehydration by refluxing with H2SO4 of moderate conc. It was less effective against the terminal alcohol than
against more reactive ones such as the 1 and 2 isomers; something like 40% conversion after 1 hour, but perhaps a system could be rigged to remove the
formed olefin continuously. I can't remember the reference, but I came across it in the achives of one of the previous incarnations of the hive.
[Edited on 15-3-2010 by manimal]
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
I guess the next step is some unneeded mercuric nitrate is going to be found a use for. Take a couple of things you dont need, and lo and behold you
got something very much needed. Isnt chemistry grand?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
mr. crow: dehydration with any acid is an equilibrium, and depending on the acid used and the substrate acted on, the equilibrium may lay on either
the alkene side or in favour of the alcohol. It wouldbe strange if we used exceptionally strong bases like tBuOK or KOH when a simple reflux with
phosphoric acid would work!
Len1: I susoect you are thinking along the same lines as myself, but mercury acetate is typically used for such transformation, generally
stoichiometrically (yuck). I bet he could find some unwanted PdCl2 and CuCl too... How about oxidation to the acid, formation of the amide and
subsequent hoffman degradation; although something gives me the impression that a "biologically inactive" molecule is not desired...
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Or maybe we got it completely wrong and its going to be acetonitrile for a Ritter of a reaction ..
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
Quote: Originally posted by len1  | | Or maybe we got it completely wrong and its going to be acetonitrile for a Ritter of a reaction .. |
Why bother replying if you doubt my scientific motivation?
From my previous posts, it should have been obvious that i have unlimited access to restricted chemicals, hence it would be much easier for me to just
buy Allylbenzene than to make it. But thats just plain boring, and doesnt put the reagents that i already have to use.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
You started your post by saying you dont have access to common chemicals?
But hey, I understand, its the modern world, you have to travel back in time to find naive fools interested in science per se.
Why did I post .. for the same reason as most posters here, masterbating my ego .. to show off the funny pun on 'Ritter' I just made up
[Edited on 16-3-2010 by len1]
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
Where did you read that?
Why do i have to defend myself for performing a basic chemical transformation? If you don't like to help my cause, shut up...but don't blame me for
shit you don't know.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Quote: Originally posted by len1  | | Or maybe we got it completely wrong and its going to be acetonitrile for a Ritter of a reaction .. |
That's just plain ritticulous, stop it!
|
|
|
trilobite
Hazard to Others
  
Posts: 152
Registered: 25-2-2004
Location: The Palaeozoic Ocean
Member Is Offline
Mood: lonely
|
|
DE819400
Journal of Organic Chemistry, 31(12), 4088-92 (1966)
|
|
|
DirkDinkel
Harmless

Posts: 5
Registered: 22-6-2014
Member Is Offline
Mood: No Mood
|
|
Sorry, for digging up the old thread, but I think it's very interesting.
3-Phenylpropan-1-ol is a very interesting educt for studying the kinematics of hydride shifts.
The intermediate carbocation will have a positive formal charge located in the 1 position. Since the position 2 would be more stable one would expect
a hydride shift in favor for position 2. And since position 3 would be the most stable position for a positive formal charge in such a carbocation,
another hydride shift may occur. Perhaps even a hydride shift from position 1 to 3 may take place.
So, I would be very interested in the distribution of product quantities. And even more in the distribution of carbocation intermediates since, there
are more possibilities.
Perhaps, 3-methyl-4-phenylbutan-1-ol would be a more interesting educt to study hydride shifts. But since 3-Phenylpropan-1-ol is cheaper, I guess it's
a good start.
Perhaps one may study the distribution of carbocation intermediates by reacting 3-Phenylpropan-1-ol with acid and a nucleophile in different
concentrations, so certain adducts form in a distribution similar to the distribution of carbocation intermediates.
Perhaps somebody has another idea of an acid tolerating nucleophile for above mentioned reaction as actonitrile since one of the products would be the
acetamide of amphetamine (or simply amphetamine because with such reaction conditions a certain amount of the amide may even be cleaved by the acid),
which turns out to be a bad intermediate for a hobby chemists freedom.
The next question will be how to do the work-up and quantify the products. Well, that depends on the nucleophile. If I find an easy to handle and
cheap nucleophile, perhaps I'll do the experiment the next time I have the possibility to do it. (Unfortunately I don't have my own lab, but since I
have a good friend in my university who has access to a lab, I might do the experiment there.)
Perhaps there is somebody with a good idea for an appropriate nucleophile. 
[Edited on 22-6-2014 by DirkDinkel]
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
New Friedel-Crafts chemistry. XIX. Cyclialkylations of some phenylalkanols
Ali A. Khalaf, Royston M. Roberts
J. Org. Chem., 1969, 34 (11), pp 3571–3574
"The conditions and results of our cyclization experiments are summarized in the accompanying Table I.
3-Phenyl-1-propanol (1) and 4-phenyl-1-butanol (2) were reported by Bogert and Davidson (2) to yield polymer
and pure tetralin, respectively, upon treatment with phosphoric acid at high temperature.
We obtained a product from the former that was shown to contain a trace of indan and a little n-propylbenzene, but the
major constituents were the three isomeric phenylpropenes resulting from normal dehydration.
Confirmatory evidence for the structure of the latter isomers was obtained by catalytic reduction which converted them to n-propylbenzene, The product
from 2 was found to consist of tetralin (80%) and three lower boiling unidentified products (20%)."
Yields are low (46%) but the substrate is easily produced by electroreduction of cinnamadehyde.......... (also attached)
/CJ
Attachment: khalaf1969.pdf (512kB)
This file has been downloaded 570 times
Attachment: law1912.pdf (1.1MB)
This file has been downloaded 551 times
[Edited on 12-9-2017 by Corrosive Joeseph]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Digging old threads in The Hive, I found an interesting experiment, that worked very well to achieve the dehydration of 3-Phenyl-1-propanol to
allylbenzene and propenyl benzene.
It works by heating 3-phenyl 1-propanol with a fatty acid as oleic, palmitic, stearic, etc with some HCL as a catalizer. First occurs the
sterification and above 150ºC ( I think 200ºC would be better) the dehydration into allylbenzene and propenyl benzene.
Allylbenzene can be isomerised to propenyl benzene by heating further with KOH.
I don't need to mention both products are widely used to make amphetamines by many routes.
I attached the PDF text with the experiment below:
Attachment: Allylbenzene and propenyl benzene from 1-phenyl 3-propanol and stearic acid.pdf (2.6MB)
This file has been downloaded 593 times
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
@ ChemiPharma
Thank you for that.......... Taken from another forum -
"What I tried with limited success was esterification with fatty acids and subsequent pyrolysis or rather thermolysis.
The yield was only around 40%, with around 15% of the unwanted isomer with the inner double bond, leaving 25% terminal alkene.
The thermolysis is rather slow and needs several hours at 300*C, held at this temperature because the fatty acids would distill otherwise.
They say, the higher this temperature is, the more of the unwanted non-terminal isomer will come out.
However I think a lower temperature would make reaction time unacceptably long.
I need to add that the fatty acids were obviously impure, so yield might rise but ratio won't.
Finally I decided that esterification with stearic acid and subsequent pyrolysis works best, thoug yields are only moderate and its rather tedious."
There is also dehydration by passing over alumina.......... Attached
/CJ
Attachment: herling1966.pdf (624kB)
This file has been downloaded 583 times
[Edited on 13-9-2017 by Corrosive Joeseph]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
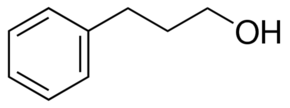
Somehow I got confused and thought the discussion was about phenyl-2-propanol, and wondered why everyone who was clearly interested in cookery wanted
to dehydrate it.
I guess this is the same stuff as hydrocinnamyl alcohol then? Like, this stuff?
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Melgar  | Somehow I got confused and thought the discussion was about phenyl-2-propanol, and wondered why everyone who was clearly interested in cookery wanted
to dehydrate it.
I guess this is the same stuff as hydrocinnamyl alcohol then? Like, this stuff?
|
Yes, that is what they were talking about. Dehydration would obviously produce an alkene, and I imagine any one of the various ways of deoxygenating
alcohols would accomplish this.
This would give a mixture of propenylbenzene and allylbenzene, both of which are known to be used in elicit manufacture, but that doesn't justify the
assumption this is an attempt at cookery.
I have an interest in conjugated double-bond reduction with NaBH4 using catalysts to prefer addition to the beta carbon, instead of the carbonyl
carbon. I was planning to use cinnamaldehyde, so I would also end up with hydrocinnamyl alcohol as one of the products.
According to the HSAB Principle, the beta carbon is a soft electrophilic center and preferentially reacts with soft nucleophiles, whereas the carbonyl
carbon is a hard electrophilic center and prefers hard nucleophiles. However, with the use of NaBH4, being a softer nucleophile, you will see
reduction of one or the other depending on which one is attacked first, but it preferentially attacks the beta-carbon through 1,4-addition and then
goes onto reduce the carbonyl group producing the saturated alcohol. If the carbonyl carbon is attacked first, then you would end up with the
unsaturated alcohol as a statistical quantity. With the use of LAH, you would see more reduction of the carbonyl carbon with a greater amount of
unsaturated alcohol.
There is some interesting chemistry here that's for sure.
[Edited on 13-9-2017 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Quote: Originally posted by Loptr  | Dehydration would obviously produce an alkene, and I imagine any one of the various ways of deoxygenating alcohols would accomplish this.
[Edited on 13-9-2017 by Loptr] |
Unfortunately, this does not seem to be the case with this particular substrate.
Acid-catalyzed dehydration of it's secondary and tertiary isomers are much reported..........
Bogert and Davidson (1934)
"General Primary Alcohol Procedure -
50cc of 85% phosphoric acid was placed in a distilling bulb(125cc), attached to a downward condenser
and distilled until a thermometer in the liquid read 230c.
10 to 40cc of the alcohol was then slowly admitted to the acid by means of a tube which ended in a
capillary reaching to the bottom of the liquid, while the internal temperature was maintained between 230-240c
Any distillate as well as the residue was then distilled with water as described above in order to separate the
volatile products.
The varied behavior of the primary alcohols employed requires the following additional details.
3-Phenyl-1-propanol.-The phosphoric acid treatment alone sufficed to bring about almost complete polymerization,
since only 2.5% of volatile material was obtained.
This boiled at 157" and yielded benzoic acid on oxidation with permanganate.
It was thus identified as 3-phenyl-1-propene.
The non-volatile residue was extracted with benzene, washed free of acid and distilled in vucuum.
About 40% of a fraction boiling at 180-190c was obtained.
It did not detolorize bromine immediately and analysis indicated that it was not di-phenpropyl ether."
Khalaf and Roberts (1969)
"Reaction of Phenylalkanols with Sulfuric Acid or Phosphoric Acid .-
The procedures described by Bogert and co-workers were essentially followed.
The only modification was in the manner in which the products were obtained.
The distillate and residue from each experiment were combined, diluted with water, and extracted with ether.
The ether layer was separated, washed with 10% sodium carbonate solution followed by water,
dried over anhydrous sodium sulfate, and finally distilled.
The results obtained are summarized in Table I."
Different work-up....... Different product........ Hardly
Taken from another page -
"The boiling point of phosphoric acid is 158 °C (316 °F; 431 K)
When phosphoric acid is above 213 °C (415 °F; 486 K), it will decompose slowly."
By the time these dudes are dripping in the alcohol the H3PO4 has already began to decompose.
It is also known that primary alcohols begin to dehydrate at about 170 to 180degreesC.
I'm sure there is room for improvement here. Needs exploring.
Interesting chemistry alright.........
/CJ
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Taken from somewhere else.........
PROPENYLBENZENES FROM PHENYLPROPANOLS
"Phenylalkenes such as propenyl benzene can be created by the dehydration of phenyl-1 -propanols.
This reaction is carried out by heating 1-phenyl-1-propanol with alumina (aluminum oxide) or vermiculite.
3-Phenyl-1-propanol will produce allybenzenes (I'm not so sure about this but here it is anyway)
Propenylbenzene from Phenyl-l-propanol
A) By Boiling with Alumina:
1-Phenyl-1-propanol (0.25 moles) is mixed in a distillation apparatus with 75 g. alumina and a pinch of hydroquinone
(p-dihydroxybenzene) or pyrogallol (inhibitor).
The mixture is heated under a vacuum for 1 hour at 150 degrees.
The mixture is washed, in a separatory funnel with a dilute solution of sodium hydroxide and then water.
The water insoluble layer is dried over anhydrous calcium chloride, sodium sulfate or magnesium sulfate.
85 % yields of propenylbenzene are obtained.
Starting Molecule: 1-( 4-Methoxyphenyl)-1-propanol
Product: Anethole; (4-Methoxypropenylbenzene)
Reference: (Muller 1957)
B) By Heating With Vermiculite:
0.25 Mole of 1-phenyl-1-propanol is heated at 90 degrees for five minutes (under reduced pressure) with 3 mL of
concentrated sulfuric acid on 8 grams of vermiculite.
The mixture is cooled and washed with a dilute solution of sodium hydroxide and water.
The water insoluble layer is dried.
Starting Mol.: 1, 1-Dimethyl-2-hydroxy-2-(p-methoxyphenyl)ethane
Product: 1, 1-Dimethyl-2-(p-methoxyphenyl)ethene
Reference: (Bruce 1952)
C) By Heating With Potassium Bisulfate:
Starting Molecule: 1-( 1 ,3-Benzodioxol-5-yl)butan-1-ol
Product: 1-(1 ,3-Benzodioxol-5-yl)butene
Reference: (Nichols 1985)"
And.......
"Allybenzene From 3-Phenyl-l-propanol By Thermal Dehydration
3-Phenyl-l-propanol(Ph-CH2-CH2-CH2-0H) to Allybenzene(Ph-CH2-CH=CH2)
Pyrolysis Apparatus
A quartz or high temperature Pyrex tube (e.g. combustion tubing), approximately 2 mm thickness, 20 mm in diameter,
one foot long is used in this apparatus.
Ten inches of the tube is filled with activated alumina (8 to 14 mesh) which is held in place with wire gauze
and a metal spring.
An iron-constant thermocouple lead is placed in a glass tube which is positioned half way up on the outside of the tube.
The entire tube is heated by a Nichrome wire or heating tape wrapped around it and regulated by a variable transformer.
The receiving flask is connected to a vacuum outlet and is immersed in a dry-ice-alcohol bath.
One mole of the propanol containing a pinch of pyrogallol (inhibitor) is placed in a dropping funnel at the top of the
column and slowly dripped into the column at a rate of one drop per second.
The temperature is maintained at 300 degrees under a vacuum of 20-25 mm. (water aspirator).
The reaction may be carried out at atmospheric pressure, but the top of the column must be closed to force the vapors down the
column.
A yellow liquid separates from the water formed during the dehydration.
The yellow liquid is washed with dilute sodium hydroxide and water or aqueous sodium carbonate to remove any adhering
inhibitor.
The product is then dried over anhydrous calcium chloride or magnesium sulfate. Yields 80 to 85 %.
Starting Molecule: p-Ally-B-phenethyl alcohol
Product: p-1-Propenylstyrene (p-Propenylvinylbenzene)
Reference: (Overburger 1951, 1954)
Starting Molecule: Hydrocinnamyl acetate
Product: Allybenzene
Reference: (Fort 1955)
Starting Molecule: p-Methoxy-hydrocinnamyl acetate
Product (Chemical Name): p-Methoxy-allybenzene
Product (Common Name): Estragole
Reference: (Fort 1955)"
Full refs available on request..........
/CJ
[Edited on 19-10-2017 by Corrosive Joeseph]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by Corrosive Joeseph  | [/rquote]
Taken from another page -
"The boiling point of phosphoric acid is 158 °C (316 °F; 431 K)
When phosphoric acid is above 213 °C (415 °F; 486 K), it will decompose slowly."
By the time these dudes are dripping in the alcohol the H3PO4 has already began to decompose.
It is also known that primary alcohols begin to dehydrate at about 170 to 180degreesC.
I'm sure there is room for improvement here. Needs exploring.
Interesting chemistry alright.........
|
When phosphoric acid decomposes from heat, it is giving off water and becoming an even more powerful drying agent. It could be that the decomposition
of the phosphoric acid is vital, or at least helpful, to the process.
Idle speculation below...
Might even be that the decomposition of the phosphoric acid is more important than the high temperature itself, in which case making polyphosphoric
acid first to use in the reaction instead of manufacturing it in situ might allow somewhat lower reaction temperatures.
[Edited on 20-10-2017 by SWIM]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Sorry to jump in....But I have a real stupid question.
I use vermiculite for various things like insulation, heating baths instead of sand etc. to me its a completely inert mineral used to insulate
houses..........
So does heating vermiculite make it react?? Or more precisely, i take it from the above that vermiculite is not the completely inert stuff i thought
it was?
Only asking because its cheap and I use it for loads of odd things, if it isnt completely inert as i thought, then maybe I might just be more careful
how and where i use it!
As a side note i also thought aluminum was fairly bland until recently!!
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
its Alumina , not aluminum 
| Quote: | | So does heating vermiculite make it react?? Or more precisely, i take it from the above that vermiculite is not the completely inert stuff i thought
it was? |
it does not react.It acts the same way as alumina,like a bed on which the alcohol lies down so that it can get a good grip on the OH molecules.Then it
rips them out and the H+ flies away as H2.It can do this because it has a lot of empty spaces(valencies) in its structure which form
temporary bonds(intercalates) with the OH.The spaces increase on heating due to expansion and since its hydrous,the water gets kicked out freeing up
more space.Its kind of like waxing actually,where the skin is the compound you want to convert to alkene,the hair the OH and the tape the "bed"  .Even the name of this phenomenon is a term used in skin care - exfoliation .Even the name of this phenomenon is a term used in skin care - exfoliation 
[Edited on 20-10-2017 by CuReUS]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  |
its Alumina , not aluminum 
| Quote: | | So does heating vermiculite make it react?? Or more precisely, i take it from the above that vermiculite is not the completely inert stuff i thought
it was? |
it does not react.It acts the same way as alumina,like a bed on which the alcohol lies down so that it can get a good grip on the OH molecules.Then it
rips them out and the H+ flies away as H2.It can do this because it has a lot of empty spaces(valencies) in its structure which form
temporary bonds(intercalates) with the OH.The spaces increase on heating due to expansion and since its hydrous,the water gets kicked out freeing up
more space.Its kind of like waxing actually,where the skin is the compound you want to convert to alkene,the hair the OH and the tape the "bed"  .Even the name of this phenomenon is a term used in skin care - exfoliation .Even the name of this phenomenon is a term used in skin care - exfoliation 
[Edited on 20-10-2017 by CuReUS] |
Thx for the info much appreciated. I somehow dont want to know how you know so much on waxing though  . .
And yes turns out Aluminum can get pretty lively if you poke it enough  . when
melted it dosnt play nice with water . when
melted it dosnt play nice with water
[Edited on 20-10-2017 by NEMO-Chemistry]
|
|
|
| Pages:
1
2 |