Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Friedel-Crafts acylation
Yesterday I performed this named reaction to obtain a small amount of acetophenone. The acylating agent was acetic anhydride. Lewis acid was
anhydrous AlCl3. Following Brewster my set-up included a safety trap and an inverted funnel over water to capture HCl gas.
During cleanup I inadvertantly got a whiff of the gas in the safety trap. It was indeed HCl! My question is: how is this HCl generated, ie, by
what mechanism?
Note: I understand where the acetic acid byproduct comes from, just not the HCl.
[Edited on 3-4-2010 by Magpie]
[Edited on 3-4-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Congratulations, Magpie! You have confirmed the mechanism of Friedel-Crafts acylation.
They do indeed give off HCl, the reason Brewster calls for a trap.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This is the reaction stated by Brewster, Exp 66:
C6H6 + Ac2O ---> C6H5COCH3 + CH3COOH
with no HCl by-product indicated.
I presume the mechanism includes -OOCCH3 bonding to the AlCl3, which then breaks down to CH3COOH after receiving a proton from the phenyl ring. If
this is true where does the HCl come from?
I'm probably oversimplifying the mechanism and the AlCl3 complex breaks down to some HCl also?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
It is the reaction of AlCl3 (actually AlCl3 coordinated with organic reaction components) with the protic reaction products, in this case mostly
acetic acid. In their interaction AlCl3 is an acid and acetic acid is a base (speaking about their interaction roles - not relative to their reaction
with H2O as a base, as the acid/base terms are commonly used). Their acid/base reaction is:
MeCOOH + AlCl3 <=> H[AlCl3(OOCMe)]
However, H[AlCl3(OOCMe)] can dissociate also in another way:
H[AlCl3(OOCMe)] <=> AlCl2(OOCMe) + HCl
Of course, this is just a terrible simplification of a solvolysis reaction of AlCl3. All these species are involved in numerous equilibrium
relationships and true solvolyses are by definition terribly promiscuous reactions and very hard to frame in simple equations and simply defined
species (in solvolyses the solvent is always everywhere and always involved). Here the difference is in the dilution of MeCOOH.
In short, AlCl3 reacts quite readily with protic basic enough solvents and even though acetic acid is much less basic than water (where AlCl3
solvolyses/hydrolyses completely), some solvolysis nevertheless occurs with carboxylic acids as well. Usually you only notice this when HCl escapes
from the system as a gas, because otherwise reversibility is granted by the equilibrium constants.
You can make a simple experiment (on a small scale!). Add some AlCl3 to each glacial acetic acid, ethanol and water (carefully!). HCl is soluble in
all these solvents, so besides some exotherm, there should not be much gas evolution. Then try the same using diluted solutions of acetic acid,
ethanol and mixture of H2O in some nonpolar solvent where HCl is not particularly soluble (CH2Cl2, hexane...). Use 1:1 ratio of AlCl3 vs. base
(MeCOOH, EtOH, H2O). You should notice the HCl gas evolution it is all about its solubility and that is actually the only reason why you even noticed
it.
[Edited on 3/4/2010 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nicodem thank you for that detailed explanation! I can see now why the subject was just not brought up in the references I consulted.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I recently made acetophenone using the procedure in Vogel’s 3rd (forum library). Vogel stated that the expected yield was 27g. All I managed to
get was 11.7g. Here’s some pictures from this experiment:

Addition of acetyl chloride
Attachment: phpD693Wv (89kB)
This file has been downloaded 1093 times
55° hold for 1 hour

Rxn product acetophenone (colored with tar)

5% caustic wash separation

Water wash separation
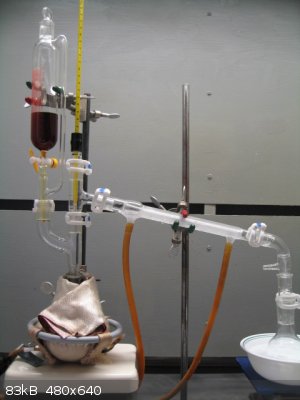
Benzene recovery

Aceotophenone coming over at 200°C
Discussion
This is the 3rd Friedel-Crafts acylation that I have done. The 1st was to make p-methylacetophenone from toluene in a school lab. The two others
were done in my lab.
Attached is an EXCEL spreadsheet comparing the 3 runs. The first procedure is clearly superior in that the yield is much higher at 79%. Based on
these results I will be using high ratios of AlCl3 and acylating agent for any of my future runs. I don’t see how Vogel managed to get such a high
yield, 60.8% based on the acylating agent.
Attachment: Friedel-Crafts comparisons of yield.xls (23kB)
This file has been downloaded 458 times
Please share the experiences you have had running Friedel-Craft acylations, especially with regard to yield efficiency.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Magpie,
With regard to yields reported in Vogel: This book is simply an aggregation of syntheses and procedures reported elsewhere, such as Organic Syntheses.
So the yields reported in Vogel depend on where the author obtained the procedure. I have found on several occasions that yields reported in Vogel are
often hard to match. Don't misinterpret me though regarding the book's overall value. Vogel is an excellent reference with lots of clearly organized
information.
And as usual, your work is very nicely reported and something I always look forward to reading.
AvB
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you AvBaeyer.
I feel chagrined that I just now noticed the fine write-up of Mercedesbenzene in Prepublication. There he reports an efficiency of 71.8% on acetic
anhydride using a Vogel procedure.
--------------------------------------------------------------------------------
For comparison I added the results of Mercedesbenzene to my EXCEL spreadsheet. His parameters do not show high ratios of AlCl3 and acylating agent to
the substrate. I now believe that his meticulous attention to water exclusion and purity of benzene gave his high yield.
Attachment: Friedel-Crafts comparisons of yield.xls (23kB)
This file has been downloaded 458 times
----------------------------------------------------------------------
I now think that there is a good chance that the low yields I show on my spreadsheet were due to poor quality AlCl3. I was using it out of my old
Daigger bottle. I had replaced this with a bottle from Elemental Scientific which performed very well when I made benzophenone (72.3% yield). But
the Elemental supply was ruined due to water vapor permeation through the plastic container so it had to be disposed (that was quite an ordeal in
itself). So I used the Daigger supply, forgetting why I had replaced it in the first place!
[Edited on 5-8-2015 by Magpie]
[Edited on 5-8-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
softbeard
Hazard to Self
 
Posts: 69
Registered: 23-7-2013
Member Is Offline
Mood: moody
|
|
Quote: Originally posted by Magpie  |
I now think that there is a good chance that the low yields I show on my spreadsheet were due to poor quality AlCl3. |
Yes, shame about water-permeation ruining your AlCl3. This has probably been answered many times, but would AlBr3 or
AlI3 also work for Friedel-Crafts? Perhaps AlBr3 or AlI3 could be prepared in-situ by reacting Al metal and the
halogen; possibly in a suitable inert solvent (like a saturated hydrocarbon, eg. hexane). Then you could be pretty sure your AlX3 is
anhydrous.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
The AlCl3 (250g) I had bought from Elemental Scientific was excellent material, very active. I used it for making benzophenone shortly after
receiving it, and it was "dynamite."
I had the bottle protected by 4 layers of plastic. The 1st layer was heavy plastic as supplied by Elemental. In the outer bag I had placed some
NaHCO3. Then it sat in my garage for ~ 6 months before I attempted to use it to make some acetophenone. This is when I discovered that it had been
ruined by atmoshperic moisture permeating the plastic bottle in which I received it. I live in a dry climate. The bottle barely had any integrity left
and it had turned black contaminating the AlCl3.
For disposal I filled a 5-gallon plastic bucket with water and ice-cubes. Then, in my hood, I slowly added the AlCl3 powder, a spoonful at a time,
stirring with a wood stick. This was a slow, noxious job as the AlCl3 still packed quite a wallop. A lot of nasty fumes were generated.
I will be getting a new supply of AlCl3 from Elemental. I hope it comes in a better container - a glass jar seems best. But there is still the
matter of the lid and seal materials being vulnerable.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
I applaud Elemental for being able to supply many extremely useful reagents. Unfortunately, they make far too much use of poorly sealing plastic
containers which obviously helps with shipping costs. I transfer most things I receive from them into quality glass bottles with good sealing
properties upon receipt.
I have used aluminum chloride in the past (when I was gainfully employed) from very old but well sealed glass bottles. These bottles were sealed with
many layers of Parafilm or had been dipped in wax. Sometimes the AlCl3 had a bit of yellow tinge but never black and always worked as intended. I do
not think going to the trouble of making the triiodide or tribromide is worth the effort.
AvB
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2693
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | This has probably been answered many times, but would AlBr3 or AlI3 also work for Friedel-Crafts? Perhaps AlBr3 or AlI3 could be prepared in-situ by
reacting Al metal and the halogen; possibly in a suitable inert solvent (like a saturated hydrocarbon, eg. hexane). Then you could be pretty sure your
AlX3 is anhydrous. |
Two problems occur to me: first, a lot of heat is generated by this reaction (boiling the relevant halogen); second, the aluminum must be activated in
the absence of oxygen. Most nonpolar solvents will catch fire during this reaction; you might be able to react small amounts of Al with Br2 in
chloroform.
|
|
|
FriedBrain
Harmless

Posts: 14
Registered: 10-8-2015
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Well, what a shitty first post, just deletetd it by an accident...
Another try:
I would suspend your Aluminium (I think as a FC catalyst Aluminiumfoil should be suitable) in a solvent like chloroform, that is inert (don't forget
that hydrocarbons will react a little bit, because of radical bromation). Then through an addition Funnel a solution of bromine in chloroform is
slowly added dropwise in your ice cooled and well stirred reaction mixture. Even if the bromine should react immediatly and higher temperature should
not accure, i would use an reflux condensor just to be safe.
Because I can't estimate the reaction of a dilute bromine solution with a suspension of Aluminium in chloroform, i would at first test it, so there
will be no surprise of something like any fire appearence.
For aluminium iodide as a FC catalyst I'm suspiciousm, but it's a try worth!
And because the storage and handling of anhydrous AlX3 is kind of shitty (fuming of HCl and it gets bad), the way discribed could be a good
way to get the catalyst right in your reaction flask.
And here is a post about right this topic @beginnings: http://www.sciencemadness.org/talk/viewthread.php?tid=63269
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Magpie  | The AlCl3 (250g) I had bought from Elemental Scientific was excellent material, very active. I used it for making benzophenone shortly after
receiving it, and it was "dynamite."
I had the bottle protected by 4 layers of plastic. The 1st layer was heavy plastic as supplied by Elemental. In the outer bag I had placed some
NaHCO3. Then it sat in my garage for ~ 6 months before I attempted to use it to make some acetophenone. This is when I discovered that it had been
ruined by atmoshperic moisture permeating the plastic bottle in which I received it. I live in a dry climate. The bottle barely had any integrity left
and it had turned black contaminating the AlCl3.
For disposal I filled a 5-gallon plastic bucket with water and ice-cubes. Then, in my hood, I slowly added the AlCl3 powder, a spoonful at a time,
stirring with a wood stick. This was a slow, noxious job as the AlCl3 still packed quite a wallop. A lot of nasty fumes were generated.
I will be getting a new supply of AlCl3 from Elemental. I hope it comes in a better container - a glass jar seems best. But there is still the
matter of the lid and seal materials being vulnerable. |
The 1kg listing of AlCl3 they sell seems to come in a glass bottle, or at least mine did. I also store it in a paint can filled with calcium chloride.
It still generates some positive pressure and when I open the paint can a cloud of vapor comes out, but it is still very reactive and white.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
The AlCl3 sits happily in the hood but the PCl5 is another story. I put the bottle in the fridge to limit exposure to moisture. This isn't working
judging from the nasty white crust around the bottle cap. I'm going to get some paraffin, displace any air with Ar and make a wax seal around the top.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Sounds like a good idea.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
FriedBrain
Harmless

Posts: 14
Registered: 10-8-2015
Location: Germany
Member Is Offline
Mood: No Mood
|
|
A wax seal, sounds interesting. Do you just melt the candles (or any other wax sources) in a container and then dip the top of your container in it?
I'm sceptical about the thightness auf this seal, I always used "Parafilm" if you know that, but my anhydrous Aluminiumchloride always diffuses some
HCl through it.
I would appreciate it, if you could make some photos of your seal!
[Edited on 12-8-2015 by FriedBrain]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I recently prepared acetophenone using 2 different procedures, ie, Brewster and Vogel (1.5X).
The Brewster procedure was small scale: 11.0g, 46%. The Vogel procedure was larger scale: 40.8g, 89.2%.
See yield comparison spreadsheet below:
Attachment: Friedel-Crafts comparisons of yield.xls (24kB)
This file has been downloaded 412 times
I was encouraged to try the Vogel procedure based on the high yield of Mercedesbenzene's preparation. His preparation is shown in Prepublication and
is based on a 1.5X scale of the Vogel procedure.
My preparation followed Vogel except I used 100mL of CH2Cl2 to extract the aqueous phase instead of using ether. This was a little tricky as I ended
up with aqueous and organic phases that were close in density. This wouldn't happen with ether.
Also, for the quench I used much more water/ice than Vogel. I probably used about 650g total. As Mercedesbenzene emphasizes the quench is violent
and must be done slowly with great caution.
My AlCl3 was a little messed up due to degraded plastic contamination from to a failed AlCl3 jar lid. But it didn't hurt my yield much, IMO.
There was significant interfacial crud during the washes. I removed this during the final wash with water.
Comments, questions, and suggestions are welcomed.

distilling off the DCM and benzene

acetophenone yield
Edit: I dried my benzene with CaCl2 and 3A mol sieves, but no other purification. I used a mechanical overhead stirrer.
[Edited on 11-8-2016 by Magpie]
[Edited on 11-8-2016 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Acylation of 1,4-Dimethoxybenzene
Just ran an acylation of 1,4-Dimethoxybenzene (DMB) to afford 2,5-dimethoxyacetophenone from Ac2O, AlCl3.
This was done small scale as I only had 320mg exactly of DMB, done in a test tube.
I used DCM as the solvent in hopes that it would help keep some oxygen out with its vapors.
Heres the notes:
Directly from my notebook, "320 mg of DMB (0.002316 mol) + 250 mg of Ac2O (0.002450 mol or 0.2316 mL) + 679 mg of AlCl3 (0.005095 mol) + 3.5 mL of DCM
(1.2mL for Ac2O and 2.3mL for main mixture)" for the exact amounts used. My scale is calibrated with 100 g and is accurate to 0.01g increments. The
scale has become somewhat off since my 100g calibration weight was found to be closer to 98.78g on my university scales.
Now I followed a procedure found from a university, the experiment was the acylation of anisole (methoxybenzene), and I assumed that the properties of
the compounds would allow this to work fine.
Here is the link to the website: North Central College Chemistry
In this order:
- I added in DCM (I dried the DCM quickly over MgSO4) and quickly measured and added the AlCl3.
- Next I dropped the two DMB crystals in and quickly shook the tube by flicking and swirling. By this point, the tube turned orangy-yellow.
- After measuring the weight of Ac2O, I added in DCM quickly to avoid evaporation.
- Slowly I added the Ac2O mixture with little to no effect. By this point the tube was turning brown, but with a weird yellow/green color. The
yellow/green couldn't be seen unless refluxing which allowed bubbles to form, which took on the color. The solution itself resembled a sort of FeCl3
color. I didn't suspect FeCl3 to be in my AlCl3 considering I got it from a good company (a friend gave me some Sigma AlCl3).
- After addition, I got a hot water bath going with a small beaker of water and began heating until reflux, for approx. 30 min.
- The solid AlCl3 began to dissapear and HCl was evolving off the tube in a decent but small quanitity. There was still smell of DMB but much less
than before. By 20 min the smell of DMB was very minute but still present and all the AlCl3 was pretty much gone, unless the tiny bits left over were
some salt or otherwise. After 2 NaOH and 2 NaCl washings, the DCM and 2,5-dimethoxyacetophenone was dried over MgSO4 and then filtered and boiled
down in the containing vial. After complete boiling down to the almost pure product, the final weight was 120mg which is approximately 28.8%.
In hindsight, I am assuming that the low yield was due to absolute fuck all control and possibly impatience during separations. However, I feel that
for almost no care given for this reaction, the 28.8% yield suggest the AlCl3 ratio as discuss in earlier posts warrants serious attention.
If any DMB is left over, then it is definitely within the liquid product which is very similar in structure and therefore probably has some ability to
dissolve each other. No crystals came out after boiling out the DCM.
Also something weird I noticed is that the ice/water quench was not nearly as violent which leads me to believe that either my DCM and/or my Ac2O was
very wet with moisture from the air. So I will try to distil them fresh and let them sit in a container over sieves/salts to dry.
As a final note, I am going to try this again with a different reagent since that was all I had, as well as get an IR and possibly an NMR for this
product, if my professor is in a good mood of course.
EDIT: added the image of the IR result

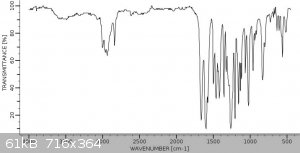
The source for the literature lookup IR: "Copyright Bio-Rad Laboratories. All Rights Reserved." LINK
[Edited on 12-7-2021 by aab18011]
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|