| Pages:
1
2 |
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
NdFeB
After searching this site to the end and google for days I finally am desperate enough to ask. Does anyone have a link or a pic to the precise
structure of Nd2Fe7B or in some places I read Nd2Fe14B? Thousands of hits to sellers which after searching their sites you learn nothing worthwhile,
and links to pay for PDF sites such as Science Direct and IOP. Why is it so hard to find for free specific structure and chemistry related information
of this super magnet material. I would really appreciate it if anyone has some decent info I could use. I want to see exactly how the atoms of these
three elements are arranged, hopefully with atom spacing data and the difference between the Fe7 and Fe14 materials. I cannot believe it is simply a
homogeneous mixture as this would not account for the anisotropy of the field alignment among other factors. I get the impression this is the case
from the way it is made (mixing the powders and heating) along with the seeming lack of relevant data on any kind of crystal structure.
Slight clarification other than some references in the pay for PDF sites to face centered cubic structure which without paying for and reading the
documents I can only assume it does form specific crystal structures (especially with the knowledge of the magnetic anisotropy). I want to understand
the relation in alignment between the Fe and Nd as well as how does the non magnetic boron figure in. I also consider the fact that under 400 degrees
it loses it magnetic properties, again implying structure. Does the Fe atoms act to allow the Nd to "broadcast" the field out of the crystal lattice,
and what role does boron play. I just cannot find a really good description of how this super-magnet material functions.
Anyone out here able to help? If so thanks in advance.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Hey,
I can't find any freely-available stuff with lattice pictures either, so you might want to give a list of the articles you want in the References
subforum, and I'll see what I can turn up. This and this looked the most interesting to me with a cursory search.
sparky (^_^)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Thanks, those look closer than what I have been going through so far. Right now I am trying to search the patent office but they have the worst search
engine. Google is not much better. I remember one time mentioning here I firmly believed pay sites came up first in google and IIRC John or maybe it
was Polverone did not agree. That was 5 years or so ago. I think I am correct even if google would not admit to it but I have found going to the last
pages in a search and working in reverse finds more free interesting stuff, in this case page 69 of the hits (25/page) yet so far nada. I think this
subject is held with a great deal of secrecy or am I just imagining things. Anyway I'll see what links I can find but the very brief descriptions,
even on the links you mention, do not indicate if what I am looking for is in there. If I come up with something I will ask you to see what you can
see. I do not know why it is but I always pick the interests which seem the most difficult to find. What started me on it is I have so many pounds of
Lanthanides I needed something new to try, being bored with glow powders and so on. Actually it was an article I read about China getting ready to
start cutting the US off in these materials due to the decreasing supply of Nd mostly due to electric cars and their increasing need. So I figure
there must be other super magnet materials and even if Nd is coming up short this does not mean I cannot make a killing creating the next super magnet
using a different rare earth. Just because China is running low on Nd does not mean they would not provide large amounts of a different element than
Nd. The hard part in playing with this subject I thought was finding ways to make powders from my incredibly hard metals but I am slowly learning the
real hard part is coming up with the science itself.
I have looked at 164 patents so far, is all you get using NdFeB as keywords. Virtually every one is about something to use the magnets for not how to
make them. Less than 10 are novel compositions where they list the whole periodic table trying to preclude others from patents, yet they are nearly
the same and not one has a clue about lattice structure. This is one damn hard bit of info to find! Below a few interesting patents, a few of them
only related to uses for, not making, NdFeB.
7,531,050
7,507,302
7,505,243
7,488,395
7,488,394
7,488,393
7,559,996
7,547,346
7,547,365
7,569,114
7,571,757
[Edited on 4-19-2010 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by IrC  | | ... Actually it was an article I read about China getting ready to start cutting the US off in these materials due to the decreasing supply of Nd
mostly due to electric cars and their increasing need. So I figure there must be other super magnet materials and even if Nd is coming up short this
does not mean I cannot make a killing creating the next super magnet using a different rare earth. Just because China is running low on Nd does not
mean they would not provide large amounts of a different element than Nd. The hard part in playing with this subject I thought was finding ways to
make powders from my incredibly hard metals but I am slowly learning the real hard part is coming up with the science itself...
|
China is not running short of neodymium. There is a likely shortage of dysprosium, used as a modifier to NdFeB magnets.
But the real excitement on this is due to China restricting exports of rare earth oxides and metals. The Chinese government has produced a number of
papers indicating they intend to become the dominant player in REE and other rare technologically significant elements as part of their mercantilist
neo-Confucianism outlook.
Part of the problem is that China produces at least 95% of the world output of REE, and their ore deposits are much larger than anywhere else. The
U.S. reserves are around 1/4 to 1/3 the size of China's.
On top of that many of the Chinese deposits are unusually rich in the heavy REE - from terbium to lutetium. These are present in much lower
percentages in most other REE deposits.
Plenty of people have been looking for replacements:
http://www.gao.gov/htext/d10617r.html
http://www.technewsdaily.com/scientists-race-to-engineer-a-n...
http://www.technewsdaily.com/us-military-supply-of-rare-eart...
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
What I meant was the US will be running low from China reducing or halting shipments. From one of your links "China has warned that its own industrial
demands could compel it to stop exporting rare earths within the next five or 10 years." says the same thing. Since I am sure green nuts will block us
from mining vast amounts of our own just as they have for 50 years forced us to send our money to the middle east for oil, we really will be running
low. I spent years prospecting around the Mt-Id border and I can see it now. They will claim mining rare earths there will trigger the super volcano
in W. Yellowstone or some such dip-shit thing like that.
Anyway, it would seem here is a project we can really discover something new as well as profit, inventing the next generation of super magnets. Still,
I cannot believe no one on earth has published crystallographic data on NdFeB as I need some or other starting point. I simply cannot find any decent
science online for this subject. I have spent so many hours searching I am going to go whacko. I knew I should have built a cabin up there in the 70's
when I was looking for U and Au. No that would not work I cannot be one of those no electricity hide from the world waiting for the end crackpots I
must have my internet and Direct TV. Hey wait, I can do it with todays technology.
If anyone is considering opening a Taco Bell up there in the wilderness let me know. A guy has got to have his limitations.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
After taking this search a bit more seriously I turned up these two. If you think these (as well as the ones I previously linked to) have what you need I'll put them up.
sparky (~_~)
P.S. I also do the "searching backwards" thing, except that I use Google Scholar. 
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Please do so this is exactly what I am looking for. Can tell more from the first "these" link in it's preview. Was hoping Miller indices would show up
somewhere and it looks like you found it.
One thing I am curious about is Fe7 and more often Fe14 being indicated. I wonder if Fe14 is really two cells linked sharing one common Nd. Having
never seen the structure but thinking about what it might look like, I picture a ring of face centered Fe atoms with Nd centered at each end. Maybe
wild guesswork but for some reason I picture the Fe ring acting like a "duct" for the fields from the Nd atoms. Of course the B gets in the way of my
imaginary pic unless it is trapped in the center of the lattice. I get stuck on the thought of the reason Boron aids the picture, some kind of
diamagnetic effect which shifts the F electrons in the Nd atoms to "align" and "duct" their field through the Fe ring which atoms of course add to the
field as the iron core in an electromagnet does. Possibly the diamagnetism of the Boron causes the high coercivity giving rise to the extreme ability
to resist demagnetization. The only other picture in my mind is two face centered rings of 4 Fe atoms, missing one which is replaced by one B atom
again with Nd at the ends. So a long chain of these cells would create a very large field.
These flights of fancy are what happens when no data is around to look at.
[Edited on 4-19-2010 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I am very sure that there must be better ferromagnetic rare-earth metals than Nd that can be separated by ion-exchange chromatography, and used as
permanent magnets, at least alloyed with Fe, Co, and/or Ni; plus a small amount of a strongly diamagnetic element like B or Al that serves to
magnetically "harden" the alloy by increasing its coercivity and retentivity (as in AlNiCo and NdFeB magnets). In fact, the best rare-earth metals for
the purpose would be those around the middle of the rare-earth series, with close to the maximum number of 7 unpaired 4f electrons and one unpaired 5d
electron possessed by Gd.
Rare-earth metals, along with Th and U, are found mostly in pegmatites, the macro-crystalline form of Precambrian granite which forms the oldest
basement rocks of continental land masses, which were the last part of the original granitic masses to cool and solidify. Apparently the rare-earth
metals (and Th and U) were about the last metals, as silicates, to crystallize out from molten granite. However, except in areas of severe erosion,
particularly by glaciation, pegmatites are at a considerable depth beneath overburdens of ordinary granite and/or much later sedimentary (or
metamorphic) rocks. Besides an area in China, such areas where pegmatites have been exposed by erosion (particularly glaciation) include parts of
Norway, Sweden, Greenland, Canada, Russia, South Africa, and Australia, so it is most unlikely that China could possibly have any monopoly on
rare-earth metals.
The metals around the middle of the actinide series, with up to 7 unpaired 5f electrons (for example Pu, which is very strongly ferromagnetic, as well
as having a maximum oxidation state of 8 with all its 5f electrons able to take part in chemical bonding), could potentially be used in permanent
magnets, but their radioactivity and especially high chemical toxicity limits this. The most accessible such ferromagnetic actinide metal would be
uranium as depleted U-238 and a half-life of 4.5 x 10^9 years, so use in permanent magnetic alloys (perhaps in alloys with Fe, Co, or Ni, and a small
amount of B or Al) could well be a way of using up the huge stockpiles of depleted U-238 that have accumulated for decades as the byproduct of
extraction of fissile U-235 as the "enriched uranium" used in bombs and the fuel rods of nuclear power plants. (The only other uses for the stuff are
as pottery glazes as the oxides, analytical chemical reagents mostly as uranyl salts, extra-dense keels for yachts where Pb is not dense enough, and
extra-heavy and hard-tipped bullets and explosive shells as used in Iraq).
[Edited on 19-4-10 by JohnWW]
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
I have at hands the ICSD, with all the structure ...
==> but currently no software to calculate the cells from that data ...
So I could in the moment only give you the crystallographic base-data, no interatomic distances or binding-angles ...
Maybe its usefule this way:
[Edited on 19-4-2010 by chief]
Attachment: ndfb.txt (4kB)
This file has been downloaded 854 times
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Still it is a starting point. I love a good mystery.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
These ORTEPs sure look interesting... 
sparky (^_^)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
see the above post for the basic data on the known Nd-Fe-B-compounds ..., uploaded a file ...
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Thanks everyone some good information. I knew the diamagnetism of the Boron was involved in the high coercivity and further thought indicates why in
my mind. Assuming the Fe rings not only aid the field but also "shield the Nd from external fields" which would reduce an outside fields ability to
shift the orientation of the Nd atoms. I can also picture a diamagnetic defect in the ring (B) which would serve to deflect external fields further
away from the lattice. Together this would answer both the high coercivity and remanence. A high BHmax along with a high Tc it seems to me could be
explained by a strongly bound ring structure acting as a shield to the Nd atoms from external forces combined with the Fe ring adding to the field. I
start thinking about the possibility of say Gd2 - 2(Unn (odd n)) - Bi. However it may be a new set of numbers is needed for a ring structure along
this line. Still, something to think about.
One other thought while sitting here looking at my floating graphite. Almost identical to this wiki pic except no hole in mine and silver (color)
plated magnets.
What about replacing the Boron with pyrolytic graphite? I cannot get this much levitation with Boron or Bismuth so obviously the diamagnetism is much
greater. Not too much of a stretch thinking about my blocks of copper graphite alloy. Except of course for the alteration of this property of carbon.
Still I wonder is there a way to use carbon with the right crystal structure in the next super magnet?
[Edited on 4-19-2010 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Pyrolytic carbon's properties are a bulk effect, the multiple interconnected graphene planes being essential. The NdFeB phases structure depends on
getting atoms into certain places to obtain certain structures that bear very little resemblance to graphene.
Pyrolytic carbon gives more levitation than bismuth because it is much less dense, around 2,2 vs 9,78. It is anisotropic so its diamagnetism varies
with the axis it is measured on.
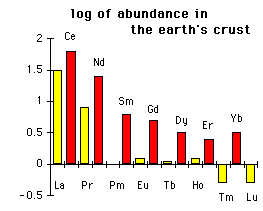
While you're busy replacing neodymium with other lanthinoids, you may want to consider the relative occurrence of them. Replacing Nd with Tm is
likely not a winning choice.
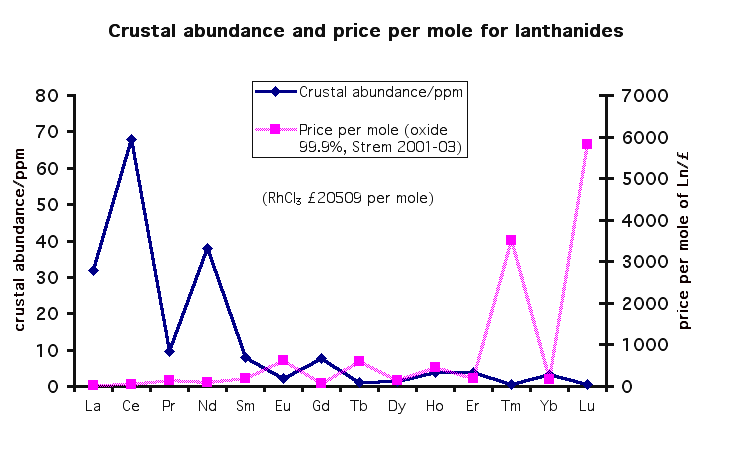
Chart in log(ppm in crust) so values below zero mean less than 1 ppm
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Yeah I figured that about the carbon but still somewhere out there is the next magical brew. I have many pounds of most of the RE's, especially La. I
imagine I will concentrate on La as it is so much cheaper than most of them. By the way thanks kmno4 for the PDF. 128 K of solid gold I tell you. I
use google too much, I really need to look into other search methods. I swear google is turning into a pay per view (meaning you pay they let others
view yours first) no doubt related to not serving up over 1000 hits even when they say there were a million or so. I can only assume the other 999,999
hits were what you were really looking for but no profit in it for google or something like that.
I was thinking the boron could be in the middle, and with a triangular structure being the strongest geometric shape it makes sense from the high
coercivity (Hci), and high Curie temperature (Tc) standpoint. I am glad I started this thread you people have really helped a great deal. Backspacing
kmno4's link to the magnets Dir contains a bunch of useful files. I would like to get better at searching, like how did kmno4 find the link to begin
with, I never ran across it searching google for keywords like NdFeB crystal structure and so forth. I miss the days when multiple engines were around
such as Northern Lights among others, the NL engine back in the late 90's always gave me more useful hits when looking for scientific subjects than
google did. Not to mention I believe google has gone the wrong way, less science and more buy it here crap than ever.
Anyway, thanks for the help people, off to really study the PDF I just received, like I said bar none so far the best structure information I have yet
encountered.
One last minute thought: http://fffff.at/dr-google/ , kmno4 I looked into this Dr Google you mentioned but I find a medical diagnosis type of engine. Is there a "science"
one? I guess I am asking how did you find the file you gave using this? From the usefulness of the PDF I think I should be using your method of
searching. Or does the medical one also search for non medical subjects?
[Edited on 4-20-2010 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
As far as I heard at university the magnetism comes from unpaired spins ... of Iron ...
==> The rest of the structure just holds the Iron in place ...
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Nevermind (Dr) Google.
Something more about "these" materials and other (M2Fe14C) can be found in "Handbook of Magnetic Materials" (search Gigapedia etc.) especially in
volume 4.
ps.
Volume 4 of this "Handbook" -->Download
|
|
|
chemoleo
|
Thread Moved
20-4-2010 at 14:51 |
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Quote: Originally posted by chief  | As far as I heard at university the magnetism comes from unpaired spins ... of Iron ...
==> The rest of the structure just holds the Iron in place ... |
You will never reach 1.4 Tesla with Iron alone. It is the F and D electrons in Nd which make Iron Neodymium Boride such a powerful magnetic material.
You must not consider unpaired electrons as the only factor. Where they are in the orbitals and the number also comes into play. Going by the number
of unpaired electrons would make one wonder why Bi is not ferromagnetic in properties, in actuality it is a diamagnet, or it repels magnetic fields. I
know, I once made a notebook with each element in the table, and showed the entire electron structure complete rather than shorthand referencing the
next noble gas below it so I could just look at the whole structure for each element. I kept seeing unpaired electrons and could not understand why so
many were not magnetic. Later on after gaining more knowledge I came to the understanding that diamagnetism was also the result of unpaired electrons
the difference being where they were in the orbital structure as opposed to other elements which had the same number or fewer electrons (speaking of
unpaired) which did show magnetic properties.
kmno4 thanks again, good information. Studying the structure I find that like snowflakes there really is art in nature, amazing to see. I find that my
thought of using La has another advantage besides being more prevalent than Nd.
However it is something I do not quite understand. It seems that making a structure comprised of rare earths and transition metals has a problem
related to where the RE is in the series. Possibly someone could clarify why this is. Is it related to the Lanthanide contraction or is it some type
of quantum effect. Going down in the series towards La gives a stronger magnet yet you would think the number of unpaired electrons would be more
important say Gd being better than La. What happens is above Sm the magnetic moments begin to couple anti parallel to the TM (transition metal)
moments, reducing the field. As we go towards the lighter RE's at Sm and "lighter", the moments begin to couple in parallel increasing the magnetic
field. This would seem to rule out everything above Sm if a very strong magnet is the goal. This is bad luck I would like to see the maximum number of
unpaired electrons in the quest for a new material. Anyway really I just wonder if anyone can explain why this is.
[Edited on 4-21-2010 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
But 1.4 Tesla is not beyond the magneztizability of "magnetic" steel-alloys (with silicon, eg.) ..., as they are found in transformers etc. ...; in
those also just the iron plays the magnetic role, while the other elements are there to make a ordering of enough iron-spins possible ...
So the iron might well count alone for most of the magnetic field ...
==> Boron, I guess, ist there for it's chemic versatility: Whoever heared anything about the chemistry of boron and saw the zoo of possible
structures knows what I mean ...
Some samarium-compounds were in developement for deep-temperature-applications, like in spaceflight ...
==> Nd is maybe just the cheapest of the usable rare-earths ...
[Edited on 21-4-2010 by chief]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
"Some samarium-compounds were in developement for deep-temperature-applications, like in spaceflight"
And no Iron is in this magnet.
On the other point you made: What I meant was permanent magnet from Iron alone with such a strong field, not what can we do like aid Iron with a coil
with current flowing through it. Iron is the bulk of metal in Nd2Fe14B. Nearly as strong is Sm2Co5, which has no Iron. You had stated all other
elements merely held the composition together and I was pointing out if this were true then why did we not have 14,000 Gauss "permanent" magnets prior
to the 20th century.
Structure as you say is important as seen from the bulk of the mixtures so far tried going from not much of a magnet to a great one merely by altering
the way treatment such as sintering is done. From this yes structure is important. I guess the point I mean is there are things going on which are
vitally important related to the actual function an "impurity" in the lattice performs, much more than mere structure. Coercivity is increased by
Boron in the structure not just because the Boron wants to be in a certain place in the lattice (but this is critical also), the diamagnetic
properties of the Boron are paramount. It sounded like you were saying "all other elements only function to create a specific lattice structure and
only Iron gives the magnetic properties". I could be wrong but this is how I read it. In any case the only point I was getting at is the magnetic
moments or diamagnetic properties are critical when considering all the other elements in the structure besides Fe.
I believe (but I could be wrong) the resistance to opposing fields weakening the magnet happens something like this. Imagine a tapered object stuck
into a say foam ring. As you go further you expand the ring until you break it. Call an external opposing field this tapered object. If you had
something pushing against this tapered object you would be making it harder for the tapered object to expand the ring thereby making it harder to
wreck the ring structure. I know we are dealing with triangular and hexagonal structures but the foam ring being spread apart seems like an easy way
to visualize what I am getting at. If the diamagnetic properties of Boron push back against this field and are orientated in such a position and
direction as to make it harder for an external field to disassociate the lattice structure (or realign the moments of the wanted field producing
elements) then it would take a greater opposing force from outside to weaken the magnet. I probably just did the worst description of how I see it
ever written yet hopefully my point that much more is being done by the non Fe elements than merely "holding the structure together" is coherent.
[Edited on 4-21-2010 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
With iron in the coil the "amplification"-effect comes from the ordering of the spins ; only that it looses this ordering as soon as the coil is
switched off ...
The NdFeB-Material just puts some sort of energy-barrier in front of any magnetization/demagnetization ... : Harder to magnetize, harder to
de-magnetize ... : Once the spins are ordered they stay in place ..., until enough activation-energy is applied to overcome the ordering, either
thermally or by a different magnetization ...
The samarium-compounds I was talking about were similar to the NdF2B-stuff, only with partially (or fully, can't remember exactly) substituting SM for
Nd ...
ICSD lists only the following 2 compounds: B4 Fe1 Sm1 , B60 Fe60 Sm17
with the parameters ....
+--------+---------------+----------+-----------+-----------+-----------+-----------+-----------+
| sgr | sum_form | a_len | b_len | c_len | alpha | beta | gamma |
+--------+---------------+----------+-----------+-----------+-----------+-----------+-----------+
| PBAM | B4 Fe1 Sm1 | 5.958000 | 11.530000 | 3.465000 | 90.000000 | 90.000000 | 90.000000 |
| P42/NZ | B60 Fe60 Sm17 | 7.098000 | 7.098000 | 58.690000 | 90.000000 | 90.000000 | 90.000000 |
+--------+---------------+----------+-----------+-----------+-----------+-----------+-----------+
Maybe this wasn't followed: I read it in the 1990s, the database is maybe 3 or 4 years old ...
Anyhow the following compounds, without Iron, are listed:
+---------+----------------------------------------+
| sgr | sum_form |
+---------+----------------------------------------+
| PBAM | B4 Fe1 Sm1 |
| P121/N1 | B5 Co1 O10 Sm1 |
| P63/MMC | B1 O3 Sm1 |
| P1 | B1 O3 Sm1 |
| P4-21C | B40 Co40 Sm11 |
| C1M1 | B6 Ca8 O20 Sm2 |
| PBAM | B4 Mn1 Sm1 |
| P31 | B6 Ge2 O34 Sm14 |
| P42/NZ | B60 Fe60 Sm17 |
| P4/MBM | B4 Sm1 |
| P121/N1 | B5 Cd1 O10 Sm1 |
| P1- | B1 O3 Sm1 |
| PM3-M | B5.76 Sm1 |
| C1M1 | B3 Ca4 O10 Sm1 |
| PM3-M | B5.82 Sm1 |
| PM3-M | B5.49 Sm1 |
| PM3-M | B5.49 Sm1 |
| PM3-M | B5.49 Sm1 |
| P6/MMM | B3 Co7 Sm2 |
| I4/MMM | C1 B2 Ni2 Sm1 |
| P6/MMM | B3 Co7 Sm2 |
| PM3-M | B6 Sm0.99 |
| P6/MMM | B2 Co3 Sm1 |
| P6/MMM | B2 Co3 Sm1 |
| PM3-M | B1 Pd3 Sm1 |
| PM3-M | B1 Rh3 Sm1 |
| P6/MMM | B2 Rh3 Sm1 |
| P6/MMM | B2 Ru3 Sm1 |
| P6/MMM | B2 Sm1 |
| P4/NMMZ | B10 Co29 Si4 Sm3 |
| P6- | B1 Ba4 N26 O1 Si12 Sm7 |
| P31 | B6 Eu3.33 Gd3.63 Ge2 Nd3.33 O34 Sm3.71 |
| I12/C1 | B3 O6 Sm1 |
| P4/MBM | B16 Si3.64 Sm10 |
| P121/N1 | B1 O17 P2 Sm7 |
| PM3-M | B0.8 Rh3 Sm1 |
| P63/MMC | B1 O3 Sm1 |
| I4/MMM | C1 B2 Rh2 Sm1 |
| PBCA | B1 O10 Si2 Sm3 |
+---------+----------------------------------------+
They contain Co and other well known stuff instead of the Fe ... ; can you identify the magnetic ones ? 
[Edited on 21-4-2010 by chief]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Your first line is merely simple high school physics, "everyone" knows that. So not sure what you are really saying other than repeating that Nd adds
no moment to the field. I do not agree. Nd's contribution to the field is no less than 1.4 uB and likely more but it takes time to study the documents
so far provided in this thread by others. You question at the end: are you asking me or someone to study and work out in theory each structure to
predict it's magnetic properties? I have to wonder how many man years of time in the laboratory were expended coming up with the list you provide. So
again unsure of your point.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I would say that few, if any, of those compositions listed above by Chief could possibly be usefully ferromagnetic - they nearly all contain too high
concentrations of diamagnetic and strongly electronegative elements - although most of them would be at least paramagnetic.
A drawback of lanthanide-based magnets is that they have too low a Curie temperature - the transition point from ferromagnetism to paramagnetism - for
many uses in which they are liable to be exposed to significant heating. Those of Co, Ni, and especially Fe, and their alloys, are much higher. I am
not sure about the actinide ferromagnetic metals, around the middle of the series; but I am sure that their Curie points are also much higher than
those of (the middle part of) the lanthanides, and comparable to that of Fe, Ni, and Co and their ferromagnetic alloys, because their 5f electrons can
participate in metallic conduction and chemical bonding to at least an extent similar to 3d electrons.
That being the case, I would very much like to see data on the magnetic properties of ferromagnetic alloys of actinide metals, especially of depleted
U-238 and of Pu-244 (which has a half-life of 82 million years, making it radiologically fairly safe but of course extremely chemically toxic, and
traces of it occur naturally in U ores), perhaps in combination with Fe and B or Al.
I am rather surprised at Irc's statement that La makes good ferromagnetic alloys, because in the ground state it has no 4f electrons and only one 5d
electron. It just might be that, in combination with Fe, 3d and 4s electrons (of which there are a total of 8) from Fe enter the seven vacant 4f
orbitals in La atoms.
I happen to posses a ferromagnetic alloy, in the form of a lamp standard, that contains no Fe, Co, Ni, or lanthanide metals. It appears to be made of
a ferromagnetic brassy-appearing Cu-Mn alloy, a composition range of which appears to have this property, in spite of Cu being only paramagnetic and
Mn being antiferromagnetic (unpaired 3d electron spins aligned parallel but in opposing directions). Apparently the excess numbers of 3d electrons in
the Cu are shared with the Mn atoms, enabling ferromagnetic alignment of electron spins comparable to Fe and Co and Ni.
BTW, I also wonder if any of the six platinum-group metals, or any of their alloys, are usefully ferromagnetic, although of course they are much too
valuable to be used for magnets to any significant extent. It is likely because their unpaired 4d and 5d electrons can participate in metallic
conduction and chemical bonding more extensively than 3d electrons.
[Edited on 22-4-10 by JohnWW]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Actually I did not think I stated La would be good, rather I hoped it would. Since I have a bunch of it, (and it is a cheaper substitute for Nd), and
from reading around page 142 or thereabouts of the link http://exchange.chemport.ru/index.php?mode=download&way=... given by kmno4 where the magnetic contribution increased going down the series
starting at Sm and going to La, even finding Ce is a good choice from the data. Another reason I have hopes for La, is the work in the text where they
found using raw Misch metal in it's typically produced proportions of RE's works better than Ce alone, and we know there is much La in Misch metal.
Were are not talking about that lighter flint Ce-Fe stuff, rather the normal multi RE form. They also go into great depth on your Actinide question,
and yes somewhere in there I read casually but have yet to study intently they mentioned using Platinum in one of the experiments performed with
fairly good results. Cannot quote the page on that one, maybe later after I have had time to really study it. Right now I am just on a quest to find a
good composition as I am sure a lot of others are also doing out there.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Heeeere I come to save the daaaay
Permanent Magnets
http://cas.web.cern.ch/cas/Belgium-2009/Lectures/PDFs/Bahrdt...
Magnetic Bulk Materials
http://magnet.atp.tuwien.ac.at/download/fidler_euro.pdf
Nanocrystalline Hard Magnetic Alloys
http://www.biblioteca.org.ar/libros/90057.pdf
Magnetic and Superconducting Materials
www.msm.cam.ac.uk/Teaching/PtIII/M13/M13H.pdf
Nanocomposite Magnets Master Thesis
http://www.physics.gla.ac.uk/~dtngo/Thesis/NDThe_Master_Thes...
Magnetic Properties & Origins Defined
http://www.freewebs.com/schandnithphys/Magnetic.pdf
Multiferroic Crystals & Thin Films
http://upload.wikimedia.org/wikipedia/commons/0/07/Multiferr...
_____________________
I'm curious what keywords do not produce the desired result since
that cannot then be properly termed a search. Try _
- NdFeB "unit cell"
- Magnequench "unit cell"
Below are resources from my own archived material.
Attachment: SD 9-85 Scanned 21-Apr.pdf (617kB)
This file has been downloaded 712 times
Attachment: PS 2-85 Scanned 21-Apr.pdf (568kB)
This file has been downloaded 880 times 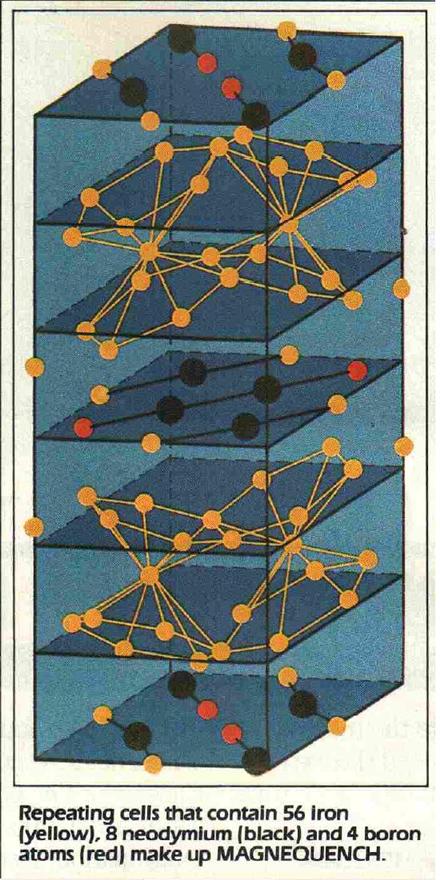
|
|
|
| Pages:
1
2 |