andre178
Hazard to Self
 
Posts: 61
Registered: 11-12-2009
Location: San Diego, CA
Member Is Offline
Mood: pacified
|
|
precipitation question
a paper calls for precipitation from methylene chloride/pentane. Is this a typical chemistry procedure? any references? I have not found anything on
google regarding this combination of chemicals, and how this would come about. Screenshot below:
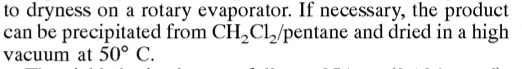
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
This simply means you have to dissolve your compound in dichloromethane and than add pentane (or petroleum ether b.p. 40-60 degC) until the compound
precipitates. This assumes that your substance is polar, so it will be soluble in dichloromethane but insoluble in an nonpolar solvent like an alkane.
This kind of purification is often used for polymers, for which the usual purification methods in organic chemistry (recrystallization, distillation,
sublimation, etc.) can't be used.
|
|
|
andre178
Hazard to Self
 
Posts: 61
Registered: 11-12-2009
Location: San Diego, CA
Member Is Offline
Mood: pacified
|
|
excellent explanation!
|
|
|