Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
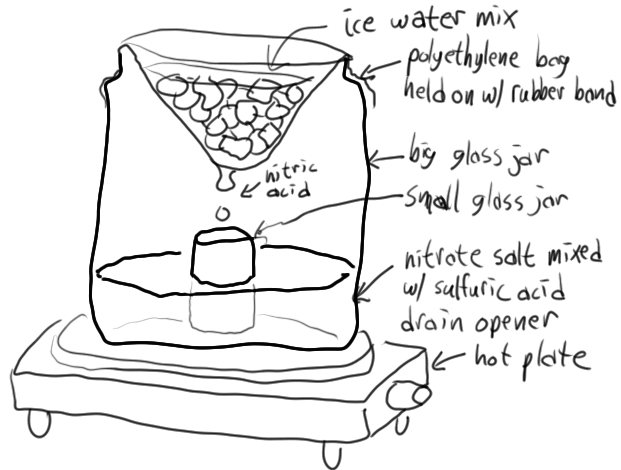
Easy nitric acid
I used to use this method before I got an actual distillation setup. It's pretty easy and gives white fuming nitric acid. It should definitely be
done outside with proper protective gear though, since if you're not careful, the plastic bag can slip and spill water into the sulfuric acid which as
we all know generates a lot of heat. For the rubber band, you need to use a really heavy one, and ideally more than one. The bag HAS to be
polyethylene. I always used translucent shopping bags from a local grocery store that was trying to encourage recycling the bags so they put this
huge recycle logo with "LDPE" (low density polyethylene) underneath it. I suppose if you wanted to make sure, you could mix a little H2SO4 and
nitrate salt (with an excess of the H2SO4) and drip it on the bag. If it doesn't discolor or damage the bag, you're probably fine. Some orange
discoloration is probably ok, since nitric acid can stain polyethylene.
Basically, the way it works, is the ice water in the plastic bag bulges down in the middle, so when nitric acid condenses on it, it tends to drip down
to the lowest point, then into the small jar. The heat needs to be carefully controlled, since if it's too hot, the nitric acid in the small jar will
boil, and if it's too cool, the nitric acid won't drip into the small jar at a fast enough rate to keep it cooler than its surroundings. Ice should
be periodically replaced as it melts, and ideally salted with NaCl (good) or CaCl2 (best) to lower the temperature of the mixture. This results in
colder nitric acid dripping into the small jar, keeping the small jar cooler, and reducing evaporation from it, thus improving yields.
In any case, I haven't seen this method described very well here, so I figured I'd lay it out. Yields won't be quite as good as with a distillation
setup, since you can't turn up the heat too high, but they're still very respectable.
[Edited on 5/26/10 by Melgar]
|
|
|
pjig
Hazard to Others
  
Posts: 136
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
nice setup.....
I'd say its as simple as it gets! I can see in a pinch how this could prove to be very useful to get small amounts of white nitric acid.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by pjig  | nice setup.....
I'd say its as simple as it gets! I can see in a pinch how this could prove to be very useful to get small amounts of white nitric acid.
|
Yeah, it definitely is. And no one that doesn't have a glassware distillation setup has any business messing around with large quantities of fuming
nitric acid anyway! 
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
This kind of method was discussed extensively at roguesci a few years ago. I used it myself at the time, but found that if you buy a large wine glass
or other appropriately shaped glass bowl, you avoid many of the problems with marginal compatibility of LDPE and HNO3. You just break off the stem of
the wine glass and you're ready to go.
Edit: Also, a small glass with stem serves very well as the receptacle as it raises the collected nitric acid out of the hot mixed acid. This reduces
refluxing of the collected nitric and thus also decomposition.
[Edited on 26-5-2010 by Microtek]
|
|
|
pjig
Hazard to Others
  
Posts: 136
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
That seems to make more sense. I guess you could take a mason jar(with nitrate and acid) and place the wine glass W/ broken stem directly on it. Fill
it with ice water and start distilling  .. ..
This would save time in the tear down, and set up.
|
|
|
MadHatter
International Hazard
    
Posts: 1332
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Nitric Acid
Looks like BarinFever's method. IIRC, temperature control was essential to obtain
white HNO3. If temperature in the reaction became too high, the result was
fuming red HNO3.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Mumbles
Hazard to Others
  
Posts: 436
Registered: 12-3-2003
Location: US
Member Is Offline
Mood: Procrastinating
|
|
I had someone recommend an old coffee maker hot plate as a heat source for this method. I saw pictures of it. Nearly clear nitric acid, but the
yield wasn't great and took most of the day to collect.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Yeah, I wouldn't think a coffee maker would be quite hot enough. I found that using calcium chloride ice melter let you turn up the heat and collect
quite a bit faster. The jar I used kind of had a bulge in the middle that held up the small jar so it didn't get too hot.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Been there done that. Its an improvised cold finger. They work great or small scale workings and its a shame people do not give them the attention
they should get.
As an added note to high a heat will get red Nitric acid so slow and steady wins the race
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
Funny that everyone links this method to BrainFever, when the original idea came from ex member of RS (also a member here) named simply RED. Inspired
by his work, I made my own version, but after some tests I just switch to the classic distillation setup. The method actually works, but only for
small scale distillations and with huge time & energy looses. The pic’s below are from my first setup, which was made before five or six year.
http://img541.imageshack.us/i/pic5005.jpg/
http://img718.imageshack.us/i/pic5008.jpg/
http://img190.imageshack.us/i/pic5009.jpg/
After some tests I discover that if you using some sort of isolation (aluminum foil, asbestos) on the outer side of the main container, this prevents
the condensation of the formed nitric acid on the cooler, inside surface and form condense only on the ice cold, suitable conical material. Using
sodium or potassium nitrate is also superior, because ammonium nitrate decomposes in much lower temperatures.
The setup below is also homemade, but works as good as my professional apparatus. No fancy parts or expensive and hard to improvise joints. The only
part, which I bought, was the 2 l. Florence flask, but in the past I use champagne bottles with great success too.
http://img35.imageshack.us/i/42030.jpg/
http://img517.imageshack.us/i/42073.jpg/
http://img96.imageshack.us/i/42091.jpg/
Sometimes I use it again, just because the nostalgia is too strong.
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
It's just a vqariation of the old russian way to make vodka with simple equipment ...
|
|
|
puppycock
Harmless

Posts: 11
Registered: 1-4-2010
Member Is Offline
Mood: No Mood
|
|
Info and pics   
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Whenever I've heated household glass jars over the hotplate, I've noticed they tend to explode (the bottom blows out) sooner or later. Instead of a
jar, I would use a heat resistant glass cooking pot. Axt demonstrated a very similar distillation, and he used two beakers IIRC. Aluminium is probably
more resistant than polyethylene to warm nitric acid. But then the acid would have some Al contamination. Some heat resistant glass tea pots might
work well in distillation setups alone (just connect the snout to a tube, and secure the top).
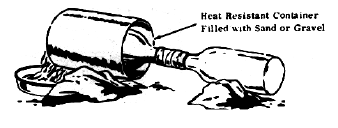
TM 31-210 describes a setup (below) with a glass bottle, using a pasty mixture of KNO3 and H2SO4 (less than 1/4 volume) to distill nitric acid. The
setup brings together two glass bottles supports the bottles with rocks with the empty bottle being slightly lower than the distillation bottle and
the receiving bottle being cooled by periodically having water poured on it. And the bottle to be heated inside a tin can filled with sand or gravel.
The setup uses paper with a tape seal (regular, electrical) to bring together the two bottles. Using paper which is sealed with tape is not a good
idea for a corrosive and oxidizing acid like HNO3, the superior thing to use is Teflon. Wrapping with aluminium foil (and then taping with electrical
tape) is another less noble option. This is not a good setup especially since overheating the heating bottle can easily burst it, wetting also does
the same. I wonder if it was even tested.
It was also pointed out in another thread by franklyn, but some older literature distills nitrate and H2SO4 in cast iron retorts. The acid strength
would need to be kept high since unalloyed and low-alloyed cast iron are said to corrode very fast in dilute or intermediate acid at all temperatures.
That's another distillation possibility.
For saftey reasons, to newbies reading this. Do not inhale nitrogen oxides! They are deadly gases that cause pulmonary edema. I have
inhaled a significant amount of them once as a careless teen and coughed up blood chunks the next day, I was lucky to not have died (coughing up blood
is one sign of pulmonary edema).
[Edited on 28-5-2010 by Formatik]
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
Just to clear things up, both of the apparatus above use sand baths. The sand itself was carefully sifted and dried before the distillations.
|
|
|
Chainhit222
Hazard to Others
  
Posts: 138
Registered: 22-8-2009
Location: peach's mailbox
Member Is Offline
Mood: grignard failing to start
|
|
I assume that the cap for that bottle is made of aluminum? It did not experience degradation due to hno3 exposure?
Another method that does not involve saran wrap or wine bottles is to use a broken wine/martini glass (the stem is snapped off). I believe Axtran
originally posted this method.
[Edited on 28-5-2010 by Chainhit222]
The practice of storing bottles of milk or beer in laboratory refrigerators is to be strongly condemned encouraged
-Vogels Textbook of Practical Organic Chemistry
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
It’s plastic cap from empty sulfuric acid bottle. Any improvisations (plugs from Teflon or aluminum foil) are also possible.
[Edited on 28-5-2010 by Jimbo Jones]
[Edited on 28-5-2010 by Jimbo Jones]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I've only ever had glass jars or bottles (of the type food or alcohol comes in) break when they were either cold and placed on a hot surface, or hot
and cooled very quickly. And then, only when they've been empty of liquids or nearly so. It's been my experience that if you have a cast iron round
heating surface on your hotplate, you can heat jars and bottles on this just fine, but if you have a coil that you can see glow, you need a sand bath
or something to even out the heat.
Whenever I've done this, I've put a cold jar on a cold hotplate, then turned the hotplate on and not removed the jar until I'm done and the hotplate's
turned off and cold. Doing it this way, I haven't had a problem yet.
|
|
|