Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Assorted Extreme Reactions
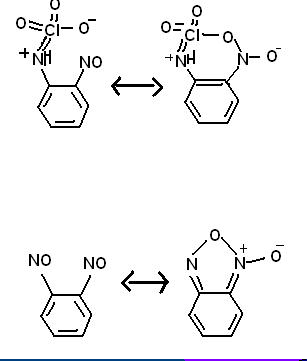
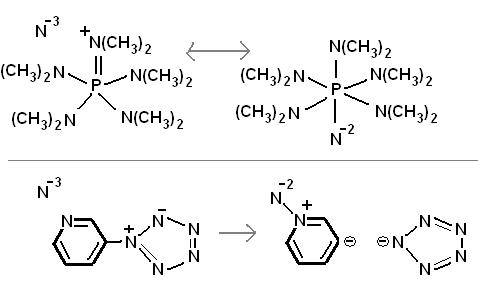
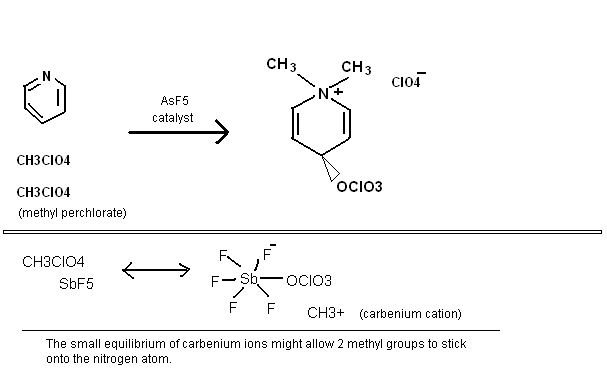
Some random reactions involving superacid equilibria, pretty self explanatory. Phenyl tetrazolate has in fact been prepared, apprently the benzene
stabilizes the 5-nitrogen ring, but the N5- anion has yet to be made. It is pretty bizarre chemistry, so I would not be surprised if some people here
think these reactions are not possible.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
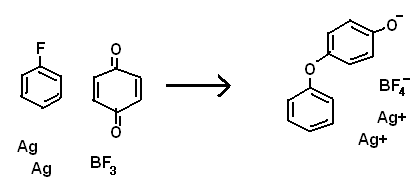
In the picture below (attached), you will find a reaction that likely will immediately make you think I don't know that BF3 is not powerful enough to
pull of a F- from benzene.
However, consider that there is probably a tiny equilibrium, and that benzoquinone might be able to effect a reaction, where otherwise there
effectively would be none.
Ag is oxidized because of entropy, not reduction potential. The reaction would probably be endothermic. The Ag+ is of course, soluble in benzene,
sticking on to the delocalized orbitals/ring.
First the fluoride is pulled off, leaving a benzene cation, then the cation oxidizes elemental silver, leaving a radical that is quickly oxidized by
the quinone, leaving another radical on the other side of the quinone, which finally oxidizes another silver atom. Thought of this last night.
Also an interesting reaction I designed:
Ba(ClO4)2 + SO2Cl2 --> BaSO4 + Cl2O6 + Cl2
The reaction is driven by the insolubility of BaSO4
In addition, Cl2O6 reacts at room temperature with fluoride to leave perchlorate and send of chloryl fluoride ClO2F gas! I am not sure if this last
reaction is endothermic.
What about swapping out the carbon atom and the extra electron in a carborane anion for a nitrogen with a positive charge and an extra F studded on?
Make it a super basic cation. Wonder if that F on the nitrogen would be reactive at all?
By the way, another stupid thought, beryllium "carboride". Wouldn't the Be+2 be a really powerful Lewis acid, since the crystal lattice energy
between the cations and anions would be so low? (As well as the carborane being highly resistant to forming covalent bonds).
So Be+2 might, under the right conditions, displace an H+ from isobutane, giving a C--Be bond, at room temp. This might make a useful first step in a
synthesis for replacing a selected H with another hydrocarbon group, (by adding CH3Br for example)
[Edited on 16-6-2010 by Anders Hoveland]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Would this reaction really occur?
Ba(ClO4)2 + SO2Cl2 --> BaSO4 + Cl2O6 + Cl2
I do have hydrated Ba(ClO4)2.nH2O (a very wet mess) and SO2Cl2. So, I could give it a try, but before doing so I'll have to make the Ba(ClO4)2
anhydrous. Is this possible by simply heating or do I distill off HClO4?
I'll try this later this evening and come back on this tomorrow. If Cl2O6 is formed, then one should easily see this, because Cl2O6 has a strong red
color.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I am not entirely sure, but I think barium perchlorate can be dried if strongly, but carefully heated. Be careful, since as you approach the
decomposition temperature, there could be an explosion. Mg(ClO4)2 is a strong dehydrating agent, but can be dried at 250degC under vacuum. However,
Mg(NO3)2(H2O) will actually give off NO2 before it gives up the water. I actually had Ba(NO3)2 and it did not pull water out from the air. By the
way, maybe try oleum on barium nitrate to get N2O5.
Be careful Cl2O6 is a very reactive oxidizer, even more so than Cl2O7. It is also a sensitive explosive and will likely give horrible burns if it
sprays up at you during an explosion, assuming that not all of it decomposes in the inital explosion.
If a single hair (or tiny speck of organic matter) falls onto it, Cl2O6 might explode. Do not use glass because of risk of scrapnel. Unless you have
teflon, I would advise against it, and bare in mind I almost never advise against even the most dangerous reactions. Another way might be Cl2O7 on
chlorate to make perchlorate and Cl2O6. Cl2O7 is much less sensitive, only about as much as Nitroglycerine! that should tell you how dangerous Cl2O6
is. And you can actually get a tiny drop of Cl2O7 on your hand if you quickly wash it off. Cl2O7 interestingly explodes on contact with iodine, but is
unreactive with sulfur! Even though sulfur is the element that is flammable.
You actually have SO2Cl2? How did you make it?
[Edited on 16-6-2010 by Anders Hoveland]
[Edited on 16-6-2010 by Anders Hoveland]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
What is this supposed to be? You know, contrary to popular belief, this is a science forum for amateurs so use the scientific method
and don't post idle fantasizing just because you are bored. There is nothing wrong in composing hypotheses if this is done properly, which means you
need to provide references in support of your ideas. You can not have a hypothesis without references! You also need to communicate comprehensively so
that the reader easily recognizes what is based on experimental or literature data, and what came out of your fantasy. Currently you are just
cluttering the forum with posts that only bring confusion, only because you are unable or do not want to write comprehensively. How is anybody
supposed to know what is part of fantasy, what is part experimental and what is literature based? If you don't know what I'm talking about, take a
break and try rereading any of your posts and you should see what I mean.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I tried the reaction with SO2Cl2 and Ba(ClO4)2. I was somewhat sceptical about this reaction, but I could easily try anyway. But indeed, the proposed
reaction does not occur.
I carefully dried the hydrated Ba(ClO4)2.3H2O and this works quite well. No decomposition at all. The crystals fall apart and a white powder is
obtained. I did the drying in a test tube on a small flame, very slowly, until no more water vapor was released. A sample of the dried solid dissolves
in water again and gives a clear solution. With a solution of KCl, a fine crystalline white precipitate is formed, this must be KClO4.
Next, I allowed the anhydrous Ba(ClO4)2 to cool down and then I added a few drops of SO2Cl2. When this is done, nothing happens. I even carefully
heated the mix and this only results in driving off vapor of SO2Cl2. No red Cl2O6 is formed.
Finally, I added some water. This makes a white slurry and nothing else. When more water is added, a turbid white liquid is obtained (due to formation
of BaSO4, the sulfate being from hydrolysis of SO2Cl2).
Actually I did not really expect a reaction to occur, otherwise I also expect this to work with NaClO4 and KClO4. But you never know, sometimes
chemistry has funny and interesting surprises, but not this time.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Thanks for doing the reaction. I do not happen to have any SO2Cl2 on hand.
A possible explanation for failure of any reaction is that BaSO4 formed a protective layer on the outside of the Ba(ClO4)2 crystals. Perhaps if the
Ba(ClO4)2 was first dissolved in concentrated HClO4 and either SO2Cl2 or HSO3Cl was added, then we could be sure that a protective layer was not
preventing reaction.
However, from what you described, I doubt even this would work. So it is probably not worth exploring further. Another thought: perhaps SO3 would
react with barium perchlorate? Of course it would not turn red, but there might be a BaSO4 precipitate formed. But how to determine if undissolved
solid was indeed BaSO4 or just undissolved Ba(ClO4)2? Maybe heat to be sure to drive off all the SO3, then take the solid left behind and see if it
dissolves in water, but heating might cause an explosion if Cl2O7 had formed in the SO3, which is a liquid.
Then, if you can obtain Cl2O7, it might react with chlorate and turn red.
Ba(ClO4)2 + SO3 --> BaSO4 + Cl2O7
I cannot imagine the above reaction not working, if the barium perchlorate is first dissolved in pure concentrated HClO4. If you do not have anhydrous
HClO4, I know of several ways to make it easily, see for example the topic: "Horribly Dangerous, but useful Reaction"
[Edited on 20-6-2010 by Anders Hoveland]
[Edited on 20-6-2010 by Anders Hoveland]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Admit it....
Your a pseudonym of The WiZard is In.... Don't BS me I have alot of practice pin pointing BS'ers
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
WiZ
References
EVERYTHING.
-Which is very handy.
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sedit  | Admit it....
Your a pseudonym of The WiZard is In.... Don't BS me I have alot of practice pin pointing BS'ers |
If you are directing this towards me, No, I am not active as "The WiZard is In". I only have one identity in the forum, and I see no reason to take on
two different names/accounts (unless I get banned at some future point). If you want references for anything specific, you can always ask; however
some of my proposed reactions have little precedent, so references are not possible, or not really applicable. When I post, I assume the reader is
familiar with various other published work pertaining to the reaction or synthesis.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by Anders Hoveland  |
Then, if you can obtain Cl2O7, it might react with chlorate and turn red.
Ba(ClO4)2 + SO3 --> BaSO4 + Cl2O7
I cannot imagine the above reaction not working, if the barium perchlorate is first dissolved in pure concentrated HClO4.
|
This works, I tried adding very finely powdered KClO4, cheaper than using Ba(ClO4)2) to conc. H2SO4, P4O10 and _slightly_ heated (I used TINY amounts,
it is dangerous!!). I'm quite sure that Cl2O7 is produced in this reaction, but no red color is observed. There is quite some white fume though. This
must be anhydrous HClO4 or Cl2O7 which reacts with water vapor. These compounds are very volatile and rather scary. I did not continue the experiment
for a long time, I quickly quenched the whole mass in a tub of water. I do not feel like preparing larger quantities of anhydrous HClO4 or Cl2O7. I
value my limbs too much 
Another nice experiment is adding KClO3 to fuming conc. H2SO4 (20% SO3). I have done this experiment on a very small scale, a tiny spatula of KClO3 in
a watch glass to which two drops of 20% oleum are added with a glass pasteur pipette. This results in formation of a red/orange liquid and also some
yellow fumes are produced. Could this be Cl2O6? If you want, I can post some pictures of this. If a piece of paper is added to this, then a purple
flash is produced and a high pitched PANG sound is produced. I can imagine that in larger quantities this is insanely dangerous. I only used 10 mg or
so.
[Edited on 21-6-10 by woelen]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Fascinating! I think the red color is indeed due to ClO2+ chloryl cations forming in equilibrium, but I do not think "real" Cl2O6 forms in any
significant quantity. The fact that your "SO3" was mostly H2SO4, was unfortunate. H2SO4 reacts with KClO3, causing decomposition. This is discussed
elsewhere on the forum, but suffice to say, the result is a little KClO4, ClO2, and KSO4H. The KSO4H unfortunately "soaks up" more of the desirable
SO3.
2KSO4H + SO3 --> K2S2O7 + H2SO4
If you want to get pure Cl2O6, which should be a dark red color, you would need to use:
Cl2O7 + KClO3 --> Cl2O6 + KClO4
Simply add a tiny ammount of dry chlorate to an excess (though still very small ammount 1mL) of liquid Cl2O7, using a test tube clamp so your hands
are not anywhere near the reaction. It is important to use more Cl2O7 than KClO3. See if a red color appears.
To obtain pure Cl2O7, you have two options. There is the usual way of carefully distilling HClO4 with P4O10, which you apparently have done. Normally
this is done under reduced pressure to reduce the heat required, but likely it can be done at normal pressure but the distillation will be extremely
slow, and even more dangerous because more heat will be required. In your experiment with KClO3, conc. H2SO4, and P4O10, the fumes were probably not
HClO4 because that has a low vapor pressure. I have read that SO3 cannot be prepared by distilling H2SO4 with P4O10, so that suggests that your fumes
were indeed Cl2O7. However, you might see if you get any fumes with only H2SO4 and P4O10. I again emphasize the danger of distillation with Cl2O7.
The other way to make Cl2O7 would be Ba(ClO4)2 dissolved in anhydrous HClO4, then reacted with pure SO3, which is a liquid. Oleum(pyrosulfuric
acid) should not be used instead. After the BaSO4 precipitates out, you will be left with a liquid containing HClO4 and Cl2O7.
(Pure SO3 must be used because Cl2O7 would react with H2SO4 to make H2S2O7 and HClO4, which you do not want)
Use a layer of benzene to extract some of the Cl2O7 from the HClO4. Cl2O7 is hydrophobic, so should be soluble in benzene. I do not think Cl2O7 reacts
with benzene at room temperature, but you might want to do a little test just to be certain before. I do not know if Cl2O6, which is more reactive,
reacts with benzene. If not, you can just add some KClO3 into the mixture of Cl2O7 dissolved in benzene. I know that N2O5 does not react with benzene,
although it does with toluene. Or use carbon tetrachloride to extract the Cl2O7 instead of benzene. This second way is safer since it does not involve
distillation.
The HClO4 with dissolved Cl2O7 in it should not be used, because HClO4 will react with KClO3, making KClO4, ClO2, Cl2, and O2. These decomposition
products should not interfere with the reaction, but the heat from the violent reaction would likely decompose any Cl2O6.
Furthermore, I think Cl2O6 reacts with H2SO4. See the reactions in "Assorted Extreme Reactions Topic". So you cannot use a mixture of SO3 in H2SO4. At
the very least, you would have to use pure H2S2O7, Ba(ClO4)2, and Ba(ClO3)2
2H2S2O7 + Ba(ClO4)2 + Ba(ClO3)2 -->
2HClO4 + 2BaSO4 + 2ClO2SO4H
Just to remind you ( I am sure you already know), Ba(ClO3)2 has advantages over KClO3.
For example, Ba(ClO4)2 + 2H2SO4 -->
BaSO4 + H2S2O7 + 2HClO4
whereas, KClO4 will not dehydrate H2SO4.
BaSO4 is insoluble; its precipitation drives the reaction to dehydration.
[Edited on 23-6-2010 by Anders Hoveland]
[Edited on 23-6-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Liquid Cl2O7 is not red. Only Cl2O6 is red. You might dissolve the Cl2O7 in lots of excess CCl4 before you react with with chlorate, which should then
turn red, because there might be an explosion if the Cl2O7 is too concentrated when it reacts.
|
|
|
Anders2
Banned
Posts: 39
Registered: 4-9-2010
Member Is Offline
Mood: No Mood
|
|
"Boron triperchlorate did not form an adduct with trimethylamine but did form a somewhat stable adduct with nitronium perchlorate. The compound is
thermally unstable, B(ClO4)3 decomposing with evolution of chlorine heptoxide"
Mosher,R. A. ; Ives,E. K. ; Morello,E. F.
THE STUDY OF BORON PERCHLORATE AND RELATED SYSTEMS
This could suggest that NO2(+) B(ClO4)4(-) is forming.
|
|
|
chemoleo
|
Thread Moved
18-9-2010 at 07:14 |
|