Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Reactions to Easily make new Precursors
These are some ideas for reactions from my big binder:
Pb(NO3)2 + SO2 ---> PbSO4 precipitate + 2NO2
the above requires a concentrated H2SO4 catalyst.
2Pb(ClO3)2 + 2 NCl3 --> 2PbCl2 + 4ClO2 + Cl2 + 2NO2
the NCl3 must be in a solvent for safety. ClO2 is explosive and a solution of it will dissolve silver.
2Pb(NO3)2 + 2NCl3 --> 2PbCl2 precipitate+ 6NO2 + Cl2
NO3- and 2Cl- and 3F- and 9H+ --> NF3, Cl2, and 3H3O+
the H+ comes from concentrated H2SO4
4NO2 and 6ClO2 --> 4NOClO4 and Cl2
the above might need to be dissolved in a solvent, and it it quite doubtful that it will take place. The whole idea is based on the possibility of a
small equilibrium of NO2 and ClO2 with NO2+ and ClO2-.
3Cl2O6+4H2SO4-->2H2S2O7+4HClO4+2ClO2
4ClO- and 2H2O2 and 4H+ --> Cl2 and 2ClO2
no chloride can be initially present
2ClO- and 2H2O2 and Cl2 --> ClO2 and H2O and 2 Cl-
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
5AgClO3 + 3I2 + 3H2O --> 5AgCl + 6HIO3
18 AgClO3 + 9Cl2 + 3I2 --> 18 AgCl + 2I2O5 +2IO2ClO4 + 16ClO2
10AgI + 10ClO2 --> 10AgCl + 4I2O5 + I2
10AgClO3 + 5Cl2 + I2 --> 10 AgCl + 2I2O5 + 10ClO2
2AgClO3 + Cl2 --> AgCl + AgClO4 + ClO2
4NH4NO3 + 4.5 Cl2 + 3ClO2 --> 4NCl3 + 6H2O + 4HNO3
4NH4ClO3 + 5Cl2 --> 4NCl3 + 2 ClO2 + 8H2O
4OCl- + H2O2 + 4H+ --> 2ClO2 + Cl2 + 4H2O
the hypochlorite must come from Ca(OCl)2; there can be no Cl- present
Cl2 + 4OCl- --> 4Cl- + 2ClO2 , pure Ca(OCl)2 must be used again
HONNOH is a real compound on wikipedia.
Ag2N2O4 + 2NF2Cl --> 2AgCl + F2NONF2 + N2O
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Must you rewrite these redox reactions - who needs it ??
It all looks like product of my last synthese....
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
4Cl2O7 + B2O3 --> 2B(ClO4)4- and 2ClO3+
perchloryl cation might be colored because of unpaired electron like ClO2+.
BF3 + CHF3 --> BF3CF3- and H+
The above is highly doubtful, but perhaps 2BF3's and a CHF3 have an intermolecular resonance, similar to how 2H2O and a singlet(excited) O2 have an
equilibrium with H2O3. CHF3 made from bromoform and AgF in DMSO or benzene.
10NaClO3 + 10NaNO2 + 8(H+) --> 4H2O + 8ClO2gas + 10NaNO3 + 2NaCl + (8Na+)
SbF5 and SO3 together, strong F- abductor than SbF5 alone? F5SbOSO2F is known to exist and does not break down to SbF6- and SO3 as might be expected.
SbF6- and ClOF3 --> -F5SbOClF4
[Edited on 18-6-2010 by Anders Hoveland]
[Edited on 18-6-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
sorry to not put it all in one big post, but it won't let me scroll down passed a certain point. I think it might be good to have a reference
available, so someone on this site might find a useful reaction. here's a superacid idea:
P(SO3F)2(SF2)F and HSO3F <--> P(OSO2F)3(SF2)F- and H+
3Na2S2O8 + 2ClO2 --> 2Na2S2O7 + 2NaClO4 + 2SO3
6KClO3 + Ca(OCl)2 + 7 H2SO4 --> 6KSO4H + CaSO4 + 4H2O + 8ClO2
2ClO2 + 2 NaOCl --> 2NaClO3 + Cl2
2NO + 2ClO3- + 2H+ --> 2HNO2 + 2ClO2-
2NO + Cl2 + 2ClO3- + 2H2O --> 2HNO3dilute + HClO4 + HCl + 2Cl-
2HClO3 + Cl2 + H2O + NO + NO2 --> ClO2 + 2HCl + 2HNO3 where the chlorine gas must be added a little at a time.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
5H2SO4 + 2NaClO3 + 3Ca(OCl)2 --> 2NaSO4H +3CaSO4 +4H2O +2Cl2 + 4ClO2
Method to make concentrated HNO3:
3 layers. 1st layer on top benzene mixed with iodine; 2nd layer in middle a very small ammount of water. The 3rd bottom layer is CCl4 solvent. First,
slowly add AgF to the benzene top layer. Secondly, a special tuble allows solid anyhydrous Ca(NO3)2 to be introduced into the water layer, bypassing
the 1st layer, and prevented from falling into the 3rd. The reactions are:
5AgF + 3I2 --> 5AgI + IF5
2IF5 + 5Ca(NO3)2 + 5H2O --> 5CaF2 precipitate + 10 HNO3anyhydrous +I2O5
The thin water layer turns into pure HNO3. I2O5 is produced and dissolved in the bottom layer.
AgI and CaF2 precipitate and fall to the bottom. 5moles of Ca(NO3)2, 10 AgF, and 6I2 are used, using a different ratio might throw off the reaction.
[Edited on 18-6-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
2SCl2 + 4NaF heat--> 4NaCl + S + SF4
4NaF + 3S heat--> 2Na2S + SF4
3SbF3 + SF4 burn--> (SbF4)2S + SbF5
SiF4gas + anyhydrous2Ca(ClO4)2 ----HClO4solvent--> SiO2 + 2Cl2O7 + 2CaF2
2NaF + thionyl chloride SOCl2 --> 2NaCl + SOF2
since PF5 is known to react similarly with NaCl
SOF2 + Ca(NO3)2 --> CaF2 + 2NO2 + SO3solid
SOF2 + Ca(ClO4)2 --> CaF2 + SO3 + Cl2O6
or 2SO3 + 2NO2 --> (NO)(NO2)S2O7 since SO3 is a very strong Lewis acid.
That would mean that SOF2 + Ca(NO3)2 would actually make NO3SO3-, NO+, and CaF2
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
NaAsF6 + BF3 --distill--> NaBF4 and AsF5vapor
since BF3 has a high boiling point.
To perform the distillation, chemically coat the glass with silver, then a nickel layer over that, either chemically of electrolyticly.
2K2MnF6 + Si(ClO4)4 --> 3KClO4 + KF + 2MnF3 + SiF4 + FOClO3
Si(OH)4 + 4Cl2O7 --> 4HClO4 + Si(ClO4)4
SiCl4 + 4NOClO4 --> 4NOCl + Si(ClO4)4
Following reaction is real: 2KMnO4 + 2KF + 10HF + 3H2O2 --> K2MnF6 + 8H2O + 3O2
MgCl2 + Ca(NO3)2 + 2AgF ---> Mg(NO3)2 anyhydrous + CaF2 + 2AgCl
The solvent used should be glacial acetic acid or acetic anyhydride. Magnesium nitrate is a strong dehydrator. It will give up NO2 before its water of
hydration when heated!
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
4IF5 + 10Ca(ClO3)2 --> 2I2O5 + 10CaF2 10ClO2 + 5Cl2O6
5AgF + 3I2 in benzene is known to make 5AgI and IF5
[Edited on 18-6-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Making N2H4:
A buret slowly drips NaOCl bleach into a beaker. The beaker has two layers, a top NH4OH with some (NH4)2SO4 dissolved in it, and a bottom hydrophobic
layer resistant to being oxidized by bleach. As hydrazine is formed, it immediately precipitates out as hydrazine sulfate (3.4g/100mL 25C) which falls
into the bottom layer, where it is protected from further oxidation by the bleach. This might allow a higher yield of hydrazine, without having to use
starch or gelatin. Ammonium sulfate solubility is 70.6g/100mL at 0C. Na2SO4 is 4.76 at 0C, not much higher than the hydrazine so you will likely get
some of this precipitating out at the bottom too.
I have so many other ideas, I cannot possibly post them all here. If you have a particular question about how to make a certain thing, ask.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
6SbF5 + 3I2 --> 5SbF4I + IF4SbF6
15SbF3 + 6IF5 --> 5SbF4I + IF4SbF6 + 9SbF5
Cl2O7 + ClO3- --> Cl2O6 + ClO4-
4Cl2O6 + (MnF6)-2 --> 2MnF3 + ClO4- + 4ClO2F + ClO4F + F-
SbCl5 + 6AgF --> AgSbF6 + 5AgCl
AgSbF6 + 4 AgF + 3I2 --> 5AgI + IF4SbF6
15 IO2SbF6 distillation, condensation --> 10 SbF4I + 5IF4SbF6 + 15O2
Cl2O7 + I2O5 --> 2IO2ClO4
or 8IO2SbF6 heat--> 2IF4SbF6 + 6SbF5 + 8O2 + 3I2
2 [IF4]2MnF6 heat--> 3IF5vapor + 2MnF3 + IF7 gas
IF7 + 3 SbF4Cl heat--> 3IF4SbF6 + Cl2 + ClF3
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
IF7 + ClF3 + 2PtF6 --> IF6PtF6 + ClF4PtF6
4(IF6+) + 2(MnF6)-2 --> 2MnF3 + IF7 + F2
4(ClF4+) + 2(MnF6)-2 --> 2ClF5 + 2MnF3 (MnF4 is not stable)
2ClF5 + 2PtF6 --> ClF6PtF6 + ClF4PtF6
3(PtF6-) + 3(ClO2+) heat--> 3PtF5 + Cl2 + ClF3 + 3O2
2PtF5 disproportionates easily into PtF4 and PtF6 (from wikipedia)
2PtF4 and IF7 burn--> PtF5 and IF4PtF6
It is extremely complicated, but if you draw a gigantic web with arrows of products becoming another reaction's reactants, you can use IF5 and Cl2O6
to indirectly make F2 and ClF5. The PtF6 is recycled. If this does not make your brain hurt, I can provide more details. Although any particular
reaction posted might not look interesting, you might notice a trend. With each reaction, the reactants become slightly more extreme products. So when
you see a reaction that generates F2 and think to yourself, "that's useless because the reactants would themselves be impossible for me to make", look
back and you will see how to make those reactants, going back a few reactions you will eventually get to easier to make reactants, until finally you
see that you could make extreme ClF5 indirectly with only really easily available reactants, if you are willing to do about ten different reactions
and separations to finally get there.
[Edited on 18-6-2010 by Anders Hoveland]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
PtF6, in the above reactions, formed by direct reaction of Pt with an excess of F2, is EXTREMELY reactive, and can be handled and stored only under
He, Ne, or Ar. It reacts readily with just about everything else, abstracting electrons from O2, N2, Xe, and possibly Kr, to form cations and PtF6-.
This was in fact how inert-gas compounds were first discovered in 1962.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
If you are willing to sort through a bunch a "trash", you will likely find several interesting reactions. Likely it will take several days to read and
comprehend all this.
4HNO3 + 14ClO2 --> 4NO2ClO4 + 4HClO4 + 5Cl2
nitronium perchlorate can be used for nitrations, but that is somewhat of a waste.
3NaClO3 + H2SO4 --> 2NaHSO4 + NaClO4 + 2ClO2
Some of the products in these reactants should be useful. If you are ever in doubt about how to make one of the precursors in a reaction, just look
back over the other reaction, and then do so again and again, until finally you get back to ordinary starting chemicals you arte more familiar with.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Anders Hoveland  |
I2 will oxidize SO2 solution to dilute H2SO4, also making HI.
HI breaks down to H2 and I2 at 425degC.
If you recycle the I2/HI, the net reaction is H2O and SO2 make dilute H2SO4 and H2 gas.
This could be useful. The dilute H2SO4 could be concentrated with SO3 in the industrial manufacture of concentrated sulfuric acid. Using dilute H2SO4,
rather than water to begin with would require less SO3 to make the final concentrated sulfuric acid. The hydrogen will also be a valuable byproduct.
|
Bunsen reaction. Old, well known. As part of the iodine-sulfur thermochemical cycle or sometimes Bunsen cycle for the production of H2 from heat, a
great deal of money has been expended on researching it to make it practical.
Attachment: pd27_pickard.pdf (737kB)
This file has been downloaded 791 times
| Quote: | | The performance of the whole S-I process is very sensitive to the composition of the two phases. Moreover, we recently highlighted that a
non-negligible amount of sulfur-containing compounds are finally dissolved in the HIx phase [2]. These sulphates (and residual SO2) must be
quantitatively removed in a downstream purification unit before subsequent operations for HI decomposition. |
Attachment: Paper160510.pdf (114kB)
This file has been downloaded 1291 times
My reply to your H2SO4 from gypsum thread was much longer than several of your burps here, others have left single postings much longer, suggesting
some sort of operator fuckup on entry.
Before further littering of the board with trivia and reactions of dubious usefulness, might you consider doing one or more of the following:
A) See if the subject has been discussed before, and if so post in that thread. Not doing so suggests an inability to read with comprehension, the
presence of yet another incarnation of Mentifex, or the products of a gleeking mammet.
B) include thermodynamic data and reaction conditions for each step, along with a discussion of known and possible difficulties. References to the
literature such as journal papers, with DOI numbers or URLs, are a plus. It too me all of 20 seconds to get those two references above, if you truly
are out to spread knowledge surely you could expend an equivalent effort.
C) include a writeup of your actually performing the reactions; amounts, conditions, equipment descriptions, work-up steps, yields, and byproducts.
See Len's papers in the Prepublication section for good examples.
D) Learn how to use the board interface properly, so as to not needlessly multiply posts as if some ego-tripping post whore.
E) take it to halfbakery.com
TIA for your cooperation.
|
|
|
stateofhack
Hazard to Others
  
Posts: 123
Registered: 6-5-2008
Location: Warm Coast
Member Is Offline
Mood: annoyed
|
|
Finally someone someone putting some common sense in this thread (Look at part "E" in the above post).
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I am sure someone will find something interesting in here and try a few of the reactions. I just had a gigantic folder of various reactions and spewed
them out as fast as I could. There was too much to give all the details to everything. Hopefully the readers have enough knowledge to fill in the
unknowns. I believe some of these reactions will be very useful and interesting to some of the readers here. Again, I apologize for not being able to
figure out how to put it in one big post. I have all these ideas and wanted to share them with a group that has at least a small chance of being
interested in them. Hopefully it is not too much information to handle.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | I just had a gigantic folder of various reactions and spewed them out as fast as I could. |
I see you're being deliberately coy about whether any of these reactions have been performed by you (or anyone else), or providing references that
would substantiate the feasibility of any of them. What does it mean that you 'had a folder of reactions'? I have the CRC Handbook on my shelf, but I
don't randomly post data from it in hopes that someone will find something interesting. If you mean that you have actually done some of these
experiments, I'm sure you'd get a lot more interest by posting some of the details. Some of the things you list (like your proposed HNO3 synthesis)
seem like 'stunt chemistry' rather than something of practical value; which frankly is the sort of thing I would applaud if someone performed it, but
as a suggestion is simply not very useful.
I don't mean to discourage you entirely as you seem to know a fair bit of chemistry (more than I do), but you seem kind of hypomanic...
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
When you put some of those equations into practice you will tend to find a different story. The thing about hypothetical ideas is half the time the
reactions don't even work. Another time they're riddled with complications.
And speaking of speculated equations generally, despite stoichiometric a reaction may not even proceed as shown because either of unknown side
reactions, or the two simply do not react (further).
Few of those equations show no merit, like this one:
2Pb(NO3)2 + 2NCl3 --> 2PbCl2 precipitate+ 6NO2 + Cl2
NO2 can be readily driven out from Pb(NO3)2 by heating alone, avoiding dangerous NCl3. Cl2 is also easily made. Assuming it were to work in the first
place, being driven by a precipitate.
Some of those interactions can be very dangerous, like this one:
3NaClO3 + H2SO4 --> 2NaHSO4 + NaClO4 + 2ClO2 (unbalanced)
This reaction is easily explosive. Some NaClO4 will form, but one risks blowing ythemselves up in the process. The reaction has been described in
literature quite a bit, and was discussed in the following thread (and also what's the much better acid to use), either way yield is low: http://www.sciencemadness.org/talk/viewthread.php?tid=11849
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
A few things you should consider
1) If someone asks a vague question about a reaction and does not provide details, journal or book references, and so on, that posting generally gets
moved to 'Beginnings' as a failure to do the needed homework before posting.
2) There has been a lot of stuff posted over the life of this site, including much speculative material. Search for a topic and add to existing
threads before just vomiting it out in a new thread.
3) One major point of this site is discussions. Puking a pile of unsubstantiated equations with no details is not too conducive to that goal.
4) Many members are relative novices, remember "amateur experimentation". They may not be able to properly evaluate the full implications of what you
give in a bare equation, nor have the proper equipment to attempt it. The use or production of compounds such as NCl3, COCl2, ClO2, and so on, with a
full discussion of the risks involved is negligent to say the least, even more so in that a number of your equations range from somewhat to highly
speculative and might not proceed as you describe.
Speculation is fine, but do it one topic at a time and provide all the background on it you have. Doing much less falls between laziness and mental
masturbation. Spewing forth great piles of trash comes close to spamming.
Let's consider a few more of these
| Quote: | 4ClO- and 2H2O2 and 4H+ --> Cl2 and 2ClO2
no chloride can be initially present
(next post)
4OCl- + H2O2 + 4H+ --> 2ClO2 + Cl2 + 4H2O
the hypochlorite must come from Ca(OCl)2; there can be no Cl- present
(duplicate, except the amount of H2O2 is different)
Cl2 + 4OCl- --> 4Cl- + 2ClO2 , pure Ca(OCl)2 must be used again |
And where does a hobbyist get pure Ca(OCl)2 ? Pool/spa supply stuff is hardly pure. So - redundant within the posts, with errors, and impractical.
Oh, and I suggest that you look up the term "singlet oxygen", here's a hint http://dx.doi.org/10.1016/j.cplett.2007.10.056
| Quote: | 4NO2 and 6ClO2 --> 4NOClO4 and Cl2
the above might need to be dissolved in a solvent, and it it quite doubtful that it will take place. The whole idea is based on the possibility of a
small equilibrium of NO2 |
Hardly seems worth the effort of typing it in then, eh? "Hey, this probably doesn't work, but why don't you try it and see, because I too lazy of
a bum to be bothered myself" is hardly the attitude that will have people paying attention to you in a favorable way.
Hey, once again I just posted more lines that you said you could. Perhaps I bribed the software, or you need to experiment a bit and ask questions.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Here is another creative, but of course completely impractical and overly-complicated, idea. It is basically a mult-step process for producing extreme
fluorine compounds.
The whole diagram would not fit into a single file [because of the sites height/width requirements] so it had to be split into two views.
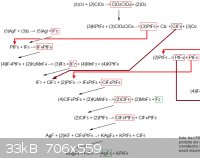 
[Edited on 14-10-2011 by AndersHoveland]
|
|
|