| Pages:
1
2
3 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by manimal  | | What do you suppose is the point of the solvent in the "normal" chlorination of styrene with Cl? Most procedures use CCl4 or similar inerts, unlike in
chlorinations of toluene. |
Can't say anything about what solvent would be ideal for your application since you told absolutely nothing about it. Solvents are certainly not
something you chose on a general basis.
If you want to prepare 1-phenyl-1,2-dichloroethane by using Cl2 via electrophilic addition (I guess this would be the most "normal" chlorination
possible), then CH2Cl2 would be the most practical choice, but you can also use other saturated halogenated solvents, ethyl acetate or other
relatively non-nucleophilic aprotic solvents. I would suggest reverse addition: adding styrene to a dichloromethane solution of Cl2 to minimize
carbocationic oligomerization. Some beta-chlorostyrene will nevertheless form as the reaction is not very selective, but a good distillation column
should separate these as well as leave the oligomers behind.
I find it surprising that you say CCl4 used as that would be the last choice of a solvent for an electrophilic chlorination (CCl4 is avoided in
synthesis unless required). Time ago someone else on this forum also said that he saw CCl4 being used for electrophilic chlorinations, but it turned
out he confused radical chlorinations with electrophilic ones (CCl4 is used as a solvent only for radical halogenations as it is inert toward Cl* and
Br* radicals).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Quote: Originally posted by Nicodem  | Can't say anything about what solvent would be ideal for your application since you told absolutely nothing about it. Solvents are certainly not
something you chose on a general basis.
If you want to prepare 1-phenyl-1,2-dichloroethane by using Cl2 via electrophilic addition (I guess this would be the most "normal" chlorination
possible), then CH2Cl2 would be the most practical choice, but you can also use other saturated halogenated solvents, ethyl acetate or other
relatively non-nucleophilic aprotic solvents. I would suggest reverse addition: adding styrene to a dichloromethane solution of Cl2 to minimize
carbocationic oligomerization. Some beta-chlorostyrene will nevertheless form as the reaction is not very selective, but a good distillation column
should separate these as well as leave the oligomers behind.
I find it surprising that you say CCl4 used as that would be the last choice of a solvent for an electrophilic chlorination (CCl4 is avoided in
synthesis unless required). Time ago someone else on this forum also said that he saw CCl4 being used for electrophilic chlorinations, but it turned
out he confused radical chlorinations with electrophilic ones (CCl4 is used as a solvent only for radical halogenations as it is inert toward Cl* and
Br* radicals). |
Yes, that is apparently true in general, but styrene is an exception in that it chlorinates well in CCl4. I have also seen a procedure that uses
hexane.
I don't really have an 'application', I'm merely curious about the reaction dynamics, e.g., whether the purpose of the inert solvent is to act as a
chlorine carrier, to minimize polymerization/ring chlorination, or what have you.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by manimal  | | Yes, that is apparently true in general, but styrene is an exception in that it chlorinates well in CCl4. I have also seen a procedure that uses
hexane. |
It is not about styrene being an exception, it is about solvent issues changing with time. CCl4 does indeed dissolve Cl2 and is inert under the
reaction conditions, so it can be used for the electrophilic addition of chlorine on alkenes. The problem is thus not in the reaction not working in
CCl4, but the properties and availability of this solvent. Once it used to be a fairly standard and relatively commonly used solvent (though it is too
non-polar for most uses as it poorly dissolves many organic compounds, at least relative to CHCl3 and CH2Cl2). Times changed a lot in the last few
decades. Just as an illustration, officially I'm not allowed to have a bottle of CCl4 in the lab where I work (though I unofficially do), even though
nobody cares if much more toxic, carcinogenic, hazardous or whatever chemicals are there. Ordering it from chemical suppliers also become a challenge
in bureaucracy, or so I was told by the person who does the ordering. Believe me that using CCl4 for something as trivial as the addition of Cl2 on
alkenes, where there are plenty of solvents that can be used instead, is just terribly weird and inappropriate (that's what I meant with "CCl4 is
avoided in synthesis unless required"). Just check the date of publication of the paper where you read about styrene chlorination in CCl4. I don't
know the reference, but I bet it is at least two or three decades old.
Even for the radical chlorination other solvents will soon put CCl4 obsolete. There is a number of solvents that could be used instead for specific
methods of radical halogenations, though none is equally general as CCl4. As you saw upthread, in the experiment of radical chlorination of toluene
with TCCA, ethyl acetate was uses as the cosolvent. You can look at this experiment also as some sort of a competitive reaction experiment. Taking it
this way, the results indicate the rate of toluene radical chlorination is about 7 times faster than the rate for ethyl acetate. So, as you see, in
this case even ethyl acetate is somewhat inert though still useless as a true solvent (if using a more diluted toluene in ethyl acetate, then the
ratio between BnCl to MeCOOCHClMe would drop to useless levels).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I did a search for chlorination using TCCA and ended up here. My project is to make phenethyl chloride and I have a number of chlorinating agents
including TCCA, SOCl2, PCl3, PCl5 and HCl. I don't seem to have ZnCl and don't want to make any. I was wondering if TCCA had been used by someone
here for chlorinating an alcohol and if so how well it worked? I think Sauron was a big fan of the stuff and dropped off some papers on it. They got
corrupted on an older drive and haven't been replaced yet in my system.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
if i'm not mistaken TCCA is a source of chlorine, like SO2Cl2, thus no good for your purpose.
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
so you guys didn't know that chlorinating toluene is the usual (old) bread and butter, standard synth for benzyl chloride? really?!
edit (next day),
there MUST be something I'm missing because a lot of you on this thread are way more knowledgeable in the field than I.
Saw the catalyst, kinda gleaned through everything. It's just that, to me, it looked like the standard route.
[Edited on 16-11-2012 by Fennel Ass Ih Tone]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Mild Aliphatic and Benzylic Hydrocarbon C–H Bond Chlorination Using Trichloroisocyanuric Acid
Sascha H. Combe, Abolfazl Hosseini, Alejandro Parra, and Peter R. Schreiner
J. Org. Chem., 2017, 82, pp 2407–2413
DOI: 10.1021/acs.joc.6b02829
| Quote: | | Abstract: We present the controlled monochlorination of aliphatic and benzylic hydrocarbons with only 1 equiv of substrate at 25–30 °C using
N-hydroxyphthalimide (NHPI) as radical initiator and commercially available trichloroisocyanuric acid (TCCA) as the chlorine source. Catalytic amounts
of CBr4 reduced the reaction times considerably due to the formation of chain-carrying ·CBr3 radicals. Benzylic C–H chlorination affords moderate
to good yields for arenes carrying electron-withdrawing (50–85%) or weakly electron-donating groups (31–73%); cyclic aliphatic substrates provide
low yields (24–38%). The products could be synthesized on a gram scale followed by simple purification via distillation. We report the first direct
side-chain chlorination of 3-methylbenzoate affording methyl 3-(chloromethyl)benzoate, which is an important building block for the synthesis of
vasodilator taprostene. |
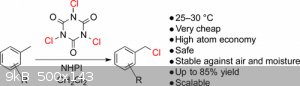
Attachment: phpnl49TW (317kB)
This file has been downloaded 776 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Nicodem,
Thanks for the link to the paper. It has some interesting mechanistic discussion but does not seem to add much to synthetic methodology. It would have
been much more useful to have information about the material balance from the various reactions. Despite it having some interesting information, I am
surprised the paper made it into JOC. I say this as a former JOC referee.
AvB
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Huh. This is just my reaction again, but instead of using a bromide salt as the bromine carrier, it uses an organobromide. The exact same mechanisms
would be in place still; the only difference is that chlorine is replacing bromine in a covalent rather than ionic bond. It would still form bromine
monochloride, which would be capable of forming radicals in the presence of longer-wavelength light (in my experience, blue light works) than chlorine
on its own. And the bromine atoms would still diffuse throughout the solution, enabling the initiation of radical chains throughout a much larger
volume, rather than just in the first few mm.
I'm curious what your thoughts on this are, Nicodem, because they're obviously greatly overcomplicating this reaction. Heck, the reason Cu(OAc)2
seems to help, could be that it selectively lets blue light through, There doesn't seem to be any indication this reaction was done in darkness, or
that there was any attempt to control ambient light. The researchers seem to be surprised by the behavior of the system in several places, but it's
not surprising at all if it's just an overly-complex variant on the reaction that I stumbled on and you tested.
I wonder if there would be any value in trying to publish a paper on this reaction now?
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
If you want to prepare 1-phenyl-1,2-dichloroethane by using Cl2 via electrophilic addition,then CH2Cl2 would be the most practical choice.I would
suggest reverse addition: adding styrene to a dichloromethane solution of Cl2 to minimize carbocationic oligomerization. Some beta-chlorostyrene will
nevertheless form as the reaction is not very selective, but a good distillation column should separate these as well as leave the oligomers behind.
|
I didn't know that adding halogen across the double bond could give b-haloalkenes as side products,so I checked it out.Apparantly,this is a
significant side reaction(41%) in this type of reaction http://www.tandfonline.com/doi/abs/10.1080/00397919808007018 (the reaction conditions are not same though) http://www.tandfonline.com/doi/abs/10.1080/00397919808007018 (the reaction conditions are not same though)
also TCCA chlorinations aren't as easy as they seem -https://youtu.be/khfYvY_Gczk?t=511 (although nurdrage didn't use ethyl acetate as co-solvent like nicodem suggested) -https://youtu.be/khfYvY_Gczk?t=511 (although nurdrage didn't use ethyl acetate as co-solvent like nicodem suggested)
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Online
Mood: Big
|
|
| Quote: | TCCA chlorinations aren't as easy as they seem -https://youtu.be/khfYvY_Gczk?t=511
(although nurdrage didn't use ethyl acetate as co-solvent like nicodem suggested) -https://youtu.be/khfYvY_Gczk?t=511
(although nurdrage didn't use ethyl acetate as co-solvent like nicodem suggested) |
NurdRage is trying to perform a Friedel-Crafts chlorination of the arene whereas Nicodem linked a radical chlorination of the side-chain. Different
reactions.
I don't know if there's a good way for FeCl3 to coordinate to TCCA to generate the electrophile needed for a Friedel-Crafts reaction. I'm having
trouble picturing how such a complex would form. In Nicodem's paper above, using GAA as solvent with TCCA promoted Friedel-Crafts reactions at the
expense of radical chlorination, but the yields of chlorotoluenes were still poor. IIRC TCCA decomposes in GAA to generate acetyl hypochlorite which is the true halogenating agent. This explains why F-C reactions only occur in GAA and
not in other solvents. I'm tempted to say that to perform a F-C halogenation with TCCA, GAA is the recommended solvent.
However, TCCA can certainly form radicals, since the N-Cl bond is weak and attacked by light. Also, once chlorination has started, TCCA reacts with
the byproduct HCl to generate Cl2, which is a much better radical former and which is probably the real chlorinating agent in the usual TCCA/toluene
reaction. When F-C chlorination is tried in an aprotic solvent, the formation of Cl2 is a bad thing because it is less electrophilic than the
chloroimine.
[Edited on 4-4-2017 by clearly_not_atara]
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
TCCA should inhibit Friedel-Crafts chlorination by complexing to Ferric chloride. NaDCC complexes with copper to form sodium copper
dichloroisocyanurate so i do not see why TCCA would not do something similar with Ferric Chloride which is a much stronger lewis acid.
Also the cyanuric acid formed in the reaction might precipitate as ferric cyanurate but im not so sure about that.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | | NurdRage is trying to perform a Friedel-Crafts chlorination of the arene whereas Nicodem linked a radical chlorination of the side-chain. Different
reactions. |
I know they are different reactions,but the separation step has to be done in both,right ? I was referring to the separation step which is the
difficult part of a TCCA reaction(from the nurdrage video)
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Online
Mood: Big
|
|
Quote: Originally posted by Σldritch  | TCCA should inhibit Friedel-Crafts chlorination by complexing to Ferric chloride. NaDCC complexes with copper to form sodium copper
dichloroisocyanurate so i do not see why TCCA would not do something similar with Ferric Chloride which is a much stronger lewis acid.
Also the cyanuric acid formed in the reaction might precipitate as ferric cyanurate but im not so sure about that. |
All Friedel-Crafts byproducts (well, almost all) inhibit the Lewis acids responsible for reaction. For example, when methyl chloride reacts with
benzene, the byproduct is HCl, which reacts with AlCl3 to form H+ and AlCl4-, the latter ion being inactive as a Lewis acid. When trichloroisocyanuric
acid transfers Cl+ to benzene, the byproduct is dichloroisocyanurate, which should react with AlCl3 to give an aluminium complex.
Note that NaDCC complexes with copper ions primarily through the negative charges on the N which is "missing" its chlorine relative to TCCA.
However, in order for that to happen, the AlCl3 has to form the FC-electrophile by complexing to TCCA and weakening the N-Cl bond to generate [Cl+],
which reacts with benzene in the first place. It's this step that I'm not so sure about (but I guess it could be ok). That the byproduct inhibits
AlCl3 is a foregone conclusion; that's why such high catalytic loadings are always used for FC reactions.
If instead you use GAA as a cosolvent, the AlCl3 can form a complex with acetyl hypochlorite which is an active FC electrophile (in fact AcOCl is so
reactive it may not need a Lewis acid), but as usual the byproduct acetate will form a complex and inhibit the catalyst.
EDIT: searching tells me H2SO4 is the preferred solvent for ring chlorination, in fact. I assume this works by a similar mechanism.
| Quote: | | I know they are different reactions,but the separation step has to be done in both,right ? |
Oh, yeah. The papers with TCCA use a solvent, whereas NurdRage uses neat toluene and tries to chlorinate most of it, which means that the TCCA-solvent
ratio is large enough to make distillation a pain.
The paper uses CH2Cl2 as a cosolvent. I think that in their case the DCCA (byproduct) dissolves in the CH2Cl2 and precipitates as slightly larger and
less colloid-like particles when the CH2Cl2 is distilled off which makes it easier for them to distill the products. Since CH2Cl2 will distill off
much faster than toluene, it doesn't seem likely that it's still there at the end of the distillation. so it must make a noticeable difference in the
consistency of the post-reaction mixture. It's also possible that the presence of FeCl3 serves to make distillation harder as this will complex with
other ions and these complexes could increase the viscosity of the solution.
EtOAc cosolvent has been reported. Acetone is also apparently a solvent for TCCA, although the production of even small amounts of chloroacetone is
very undesirable.
[Edited on 5-4-2017 by clearly_not_atara]
[Edited on 5-4-2017 by clearly_not_atara]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | | I don't know if there's a good way for FeCl3 to coordinate to TCCA to generate the electrophile needed for a Friedel-Crafts reaction. I'm having
trouble picturing how such a complex would form. In Nicodem's paper above, using GAA as solvent with TCCA promoted Friedel-Crafts reactions at the
expense of radical chlorination, but the yields of chlorotoluenes were still poor. IIRC TCCA decomposes in GAA to generate acetyl hypochlorite which is the true halogenating agent. This explains why F-C reactions only occur in GAA and
not in other solvents. I'm tempted to say that to perform a F-C halogenation with TCCA, GAA is the recommended solvent. |
First of all, calling an electrophilic aromatic chlorination a "F-C halogenation" makes no sense. Friedel-Crafts reactions are something else.
I don't know why you say that acetic acid is "the recommended solvent". I would expect a diminished regioselectivity when using it as a solvent.
Certainly an inert solvent like dichloromethane is a better choice, as in principle, direct electrophilic chlorination with TCCA should give better
para-selectivity (though obviously, even under best of circumstances, you can't get much more than 1 : 1 para/ortho selectivity on toluene). Using
sterically less demanding electrophiles (such as AcOCl) is going to reduce para selectivity. See the solvent effect evaluation in DOI:
10.1007/s00706-014-1383-6 to see how the nature of the in situ electrophile formed from TCCA affects the regioselectivity on anisole (not really
comparable to toluene, but it gives you some idea).
| Quote: | | However, TCCA can certainly form radicals, since the N-Cl bond is weak and attacked by light. Also, once chlorination has started, TCCA reacts with
the byproduct HCl to generate Cl2, which is a much better radical former and which is probably the real chlorinating agent in the usual TCCA/toluene
reaction. When F-C chlorination is tried in an aprotic solvent, the formation of Cl2 is a bad thing because it is less electrophilic than the
chloroimine. |
There is no HCl as a byproduct in the Wohl-Ziegler reactions with TCCA. And TCCA was among the haloimides used in the seminal article where this
reaction was first reported (almost a century ago). If I remember correctly, it was reported for a reaction with cyclohexene. It was only reported
much later for a benzylic chlorination of toluene.
Where do you get that Cl2 is a "much better radical former" than TCCA?
And that Cl2 is less electrophilic than the chloroimide? On the contrary. TCCA will not significantly react with toluene in the absence of
acid catalysis (I used CF3COOH in an example somewhere in this thread, but any non-oxidizable acid of suitable strength will do - using Al
or Fe chlorides does not sound like the first option to try, given that they are likely to be oxidized by TCCA). Chlorine is much more reactive.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
I decided to sloppily attempt the reaction using BCDMH as a catalyst. I left it out in the sun for a while and the solution of TCCA in toluene took on
a slightly brown color and some, what i presume is cyanuric acid precipitated, but the smell only changed a tiny bit. Benzyl chloride is supposed to
be a strong lachrymator, so what happened?
My TCCA is pretty impure, it is supposed to contain a few percent boric acid and Copper Sulfate but i do not see why that would affect it.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Since this is a chain reaction, it can be very sensitive to certain contaminants. If your TCCA has significant impurities, I recommend using it as a
source of chlorine gas instead, and bubbling that into toluene.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I would assume so, since it's used as an initiator for polymerization. Of course, you should be careful with it.
|
|
|
OrganoLeptic
Harmless

Posts: 3
Registered: 4-9-2017
Member Is Offline
Mood: No Mood
|
|
I would like to thank the posters in this thread for providing useful information (especially Nicodem).
Based on your comments and work I prepared benzyl chloride.
Toluene served both as the solvent and reactant.
Finely powdered trichloroisocyanuric acid (TCCA) was the source of the chlorine radicals.
Sunlight was used as the radical initiator.
My final yield was 28% based on the TCCA used.
You can check out the synthesis here:
https://www.youtube.com/watch?v=G_4kfY0dR2A
I have a two part question I was not able to find an answer to:
1) Do toluene and benzyl chloride form an azeotrope?
2) If they do what is its composition and boiling point?
[Edited on 4-9-2017 by OrganoLeptic]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by OrganoLeptic  | I would like to thank the posters in this thread for providing useful information (especially Nicodem).
Based on your comments and work I prepared benzyl chloride.
Toluene served both as the solvent and reactant.
Finely powdered trichloroisocyanuric acid (TCCA) was the source of the chlorine radicals.
Sunlight was used as the radical initiator.
My final yield was 28% based on the TCCA used.
You can check out the synthesis here:
https://www.youtube.com/watch?v=G_4kfY0dR2A
I have a two part question I was not able to find an answer to:
1) Do toluene and benzyl chloride form an azeotrope?
2) If they do what is its composition and boiling point?
[Edited on 4-9-2017 by OrganoLeptic] |
I typed "toluene benzyl chloride azeotrope" into Google, and discovered that indeed they do have an azeotrope, and if you want to separate them, your
best bet is to add isopropanol, distill the toluene/isopropanol azeotrope, then distill benzyl chloride once the toluene is all gone. You really
ought to vacuum distill this stuff, since impure benzyl chloride can polymerize when heated, presumably due to some sort of Friedel-Crafts alkylation.
I did this reaction again recently, and I can add some more information for anyone attempting to replicate this. First, the reaction goes much better
if you dry your chlorine before bubbling it into the toluene. Silica gel and calcium chloride have both been confirmed to work. Otherwise, the HCl
that's produced as a byproduct won't ever leave the vessel, because of its attraction to the water. This tends to manifest itself as slight cloudiness
in the reaction vessel, and a sluggish reaction.
Second, if you have elemental bromine and prefer to use that, you don't need to add it in liquid form, Even a drop is probably too much. Rather, you
can just remove the cap from your bottle of bromine, and tip it sideways a bit directly over the toluene. The orange vapors should fall directly down
and be absorbed. 3-5 seconds of this should be enough.
I've gotten this to work with a "soft white" LED lightbulb, even. With bromine present as a catalyst, it seems as though the important wavelength
range of light would be whatever can cause bromine monochloride to disassociate, but is transmitted by chlorine.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
| Pages:
1
2
3 |