| Pages:
1
..
19
20
21
22
23
24 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Fingers crossed here too, Mr HS! Very sensible approach, BTW...
|
|
|
DalisAndy
Hazard to Others
  
Posts: 129
Registered: 8-5-2015
Member Is Offline
Mood: No Mood
|
|
Hey I may have found something interesting: http://www.sciencefairadventure.com/Neodymium.aspx
And then this:
http://pubs.rsc.org/en/Content/ArticleLanding/2015/GC/C5GC00...
[Edited on 11-6-2015 by DalisAndy]
Elements Collected: 19/81 (Excluding all radioactive, using placecard for those)
Any tips or good sources are welcome.
|
|
|
DalisAndy
Hazard to Others
  
Posts: 129
Registered: 8-5-2015
Member Is Offline
Mood: No Mood
|
|
Would it be possible to use a chelating agent to either remove iron or the neodymium? I know EDTA has been used for Uranium poisoning, uranium and
neodymium are in the same column too.
Elements Collected: 19/81 (Excluding all radioactive, using placecard for those)
Any tips or good sources are welcome.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Chelating agents like EDTA are quite non-discriminating: they form strong complexes with many (most, in fact) non-alkali metals, including Fe and Nd.
U and Nd being in the same column is really a meaningless coincidence. Nd belongs to the First f-block (Lanthanides), which show very strong
inter-group chemical similarities. U belongs to the Second f-block (Actinides), which show also strong inter-group chemical similarities (but less so
than Lanthanides).
Due to their electronic configuration structures, both groups also show similarities between each other.
Similarity between U and Nd however is really quite weak. Yes, both form EDTA complexes but then so do... erm... Al and Pb! U forms oxidation states
from +2 to +6 (but +3 isn't very stable), Nd only does +3. U forms very stable complex anions and cations, Nd only forms complexed Nd(+3) cations.
By contrast, the Fe/Nd separation methods presented and tested to destruction in this thread are simple to use and require mostly only OTC chemicals.
That's why we chose them...
[Edited on 11-6-2015 by blogfast25]
|
|
|
DalisAndy
Hazard to Others
  
Posts: 129
Registered: 8-5-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Chelating agents like EDTA are quite non-discriminating: they form strong complexes with many (most, in fact) non-alkali metals, including Fe and Nd.
U and Nd being in the same column is really a meaningless coincidence. Nd belongs to the First f-block (Lanthanides), which show very strong
inter-group chemical similarities. U belongs to the Second f-block (Actinides), which show also strong inter-group chemical similarities (but less so
than Lanthanides).
Due to their electronic configuration structures, both groups also show similarities between each other.
Similarity between U and Nd however is really quite weak. Yes, both form EDTA complexes but then so do... erm... Al and Pb! U forms oxidation states
from +2 to +6 (but +3 isn't very stable), Nd only does +3. U forms very stable complex anions and cations, Nd only forms complexed Nd(+3) cations.
By contrast, the Fe/Nd separation methods presented and tested to destruction in this thread are simple to use and require mostly only OTC chemicals.
That's why we chose them...
[Edited on 11-6-2015 by blogfast25] |
would the other method work? Using CaF instead of NH3HF?
Elements Collected: 19/81 (Excluding all radioactive, using placecard for those)
Any tips or good sources are welcome.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Huh? CaF<sub>2</sub> (NOT CaF), aka Fluorite, is water insoluble.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
From Andy's first link:
"Neodymium is found most often in nature in Misch metal, monazite sand, and the mineral bastnasite. Due to the fact that these minerals and compounds
also contain lanthanides and other rare earth elements, it is difficult to isolate neodymium. The process of neodymium isolation and extraction is
highly complex; due to the complexity of the process, neodymium is never isolated on a small scale laboratory basis."
We'll see about that...
I'll show them! I'll show them all!!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
HAHAHAHHAHHHAHAHAHHHAHHHAAHHHAHAHAH!
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Totally missed that one! I was suspicious of the 'science fair' website though.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
"And here, next to my native gold and silver, is some of that native Misch metal y'all wanted to see! I bought it off an anonymous Craiglist
seller, years ago."
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quantification of legitimacy involves an index of illegitimate conjunctions and its <->it's errors within a given paper. 
|
|
|
DalisAndy
Hazard to Others
  
Posts: 129
Registered: 8-5-2015
Member Is Offline
Mood: No Mood
|
|
How would one isolate the fluoride salt from the bisulfate?
Elements Collected: 19/81 (Excluding all radioactive, using placecard for those)
Any tips or good sources are welcome.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
What fluoride from what bisulfate? I don't think anyone ever used bisulfate in this thread. Clarify what you mean.
While I'm here, I'll mention that I'm gearing up to get back on track with this experiment. I sincerely hope to have something to report by the end of
the weekend.
|
|
|
DalisAndy
Hazard to Others
  
Posts: 129
Registered: 8-5-2015
Member Is Offline
Mood: No Mood
|
|
How did you guys separate the neodymium fluoride and ammonium sulfate?
Elements Collected: 19/81 (Excluding all radioactive, using placecard for those)
Any tips or good sources are welcome.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
NdF3 is insoluble in water while (NH4)SO4 is soluble in water as well as NH4F and
Nd2(SO4)3.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
A fact you would have known had you (gasp!) READ THE THREAD.
[Edited on 8-25-2015 by MrHomeScientist]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Reading this thread takes more than the standard 8 second attention span of modern people.
Please condense it to a photo, a few smileys and an instant answer (plus a 'like' button) to make it suitable for SmartPhone users.
Something like this :-

Magnet -> Pure Neodymium   <a
href="http://www.telegraph.co.uk/news/science/science-news/11607315/Humans-have-shorter-attention-span-than-goldfish-thanks-to-smartphones.html"
target="_blank">Like</a> <a
href="http://www.telegraph.co.uk/news/science/science-news/11607315/Humans-have-shorter-attention-span-than-goldfish-thanks-to-smartphones.html"
target="_blank">Like</a>
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Haha, indeed. I'm sure the information will be condensed into a more readable format. Until then, it is the responsibility of the reader not to be
lazy. 
|
|
|
CrossxD
Hazard to Self
 
Posts: 66
Registered: 6-7-2015
Member Is Offline
Mood: stainless
|
|
maybe you can percipitate iron using aluminum you will end up with Nd III+ and Al III+ then you add NaOH aluminum will stay in solution as NaAl(OH)4
and neodymium will percipitate as Nd(OH)3......teoreticaly
[Edited on 12-2-2016 by CrossxD]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
I had to laugh when I read that as well. I have a few 1 lb ingots of it, they are amazingly clean considering they were dug up in mines. My biggest
dilemma is figuring out what process underground shapes them into such nice ingot appearing forms. Anyone know the answer to this?
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by IrC  | My biggest dilemma is figuring out what process underground shapes them into such nice ingot appearing forms. Anyone know the answer to this?
|
You're wrong. They grow on trees, actually.
|
|
|
Texium
|
Thread Topped
28-6-2016 at 18:11 |
Atrum
Hazard to Self
 
Posts: 73
Registered: 7-12-2014
Member Is Offline
Mood: Tired AF
|
|
I recently joined the fun in extracting Neodymium compounds from rare earth magnets. I have had decent success in isolating Nd sulfate, and oxalate
(the easy one).
My initial process was the sulfuric acid separation. The magnet was a small 1" cube that had no nickel plating on it. It was old and the plating had
a chip in it, and so over the last 5 years had completely come free from the plating.
Like a noob I forgot to weigh the magnet before proceeding.
1. Dissolved in 30% H2SO4. No heating was involved in an attempt to limit any possible reaction with boron. This took about a week to fully dissolve.
2. I oxidized the resulting mixture with 30% H2O2 until no further reaction could be seen.
3. I added a 2 molar solution of NaOH to the magnet soup and precipitated the insoluble hydroxides.
4. Washed the hydroxides with distilled water.
5. After filtering the hydroxides off, I reacted the brown sludge with 50% Sulfuric acid. It dissolved but it left a very fine tan precipitated of
something. I was not sure whether this was an Nd compound or an iron compound. It was completely insoluble in water. So I used some conc. HCl and
obtained a bright yellow solution. This suggests iron chloride I believe.
Based on that my guess as to what the insoluble compound was is Iron hydroxide sulfate (Fe(OH)(SO4)). I could be completely wrong about that though.
6. I tested the supernatant for iron using salicylic acid (I did not have any Potassium Ferricyanide). It was positive. I then tested for Nd using
oxalic acid. Also positive.
7. I boiled the supernatant which darkened in color upon heating which is characteristic of iron compounds being thermochromic. After removing nearly
500mL of water, I started to see some pink crystals. I filtered these off and dried them. The crystals were not quite the right color (under any
lighting conditions) and some recrystallizing is needed.
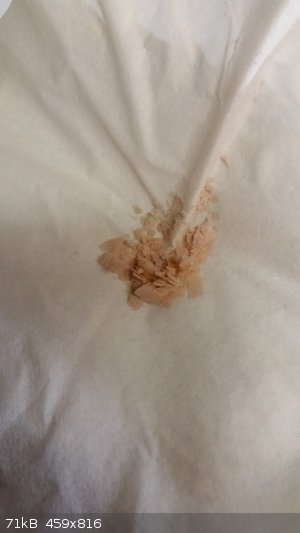 
The Nd oxalate 1 was first oxidized to remove any iron(II). The Nd oxalate 2 was not oxidized and resulted in a very yellow powder of Nd oxalate.
[Edited on 7-11-2016 by Atrum]
"Experience is my one true mistress and I will cite her in all cases. Only through experimentation can we all truly know anything." ~Leonardo da Vinci
My inventory
Recently acquired elements: Iodine , Cobalt, Tungsten, Silicon |
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@Atrum:
Welcome to the club.
|
|
|
Atrum
Hazard to Self
 
Posts: 73
Registered: 7-12-2014
Member Is Offline
Mood: Tired AF
|
|
Thanks.
I was wondering if you could provide any insight to the precipitate I mentioned in my post?
Did you come across anything like that during your processes?
"Experience is my one true mistress and I will cite her in all cases. Only through experimentation can we all truly know anything." ~Leonardo da Vinci
My inventory
Recently acquired elements: Iodine , Cobalt, Tungsten, Silicon |
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Atrum  |
I was wondering if you could provide any insight to the precipitate I mentioned in my post?
Did you come across anything like that during your processes? |
| Quote: | | 5. After filtering the hydroxides off, I reacted the brown sludge with 50% Sulfuric acid. It dissolved but it left a very fine tan precipitated of
something. I was not sure whether this was an Nd compound or an iron compound. It was completely insoluble in water. So I used some conc. HCl and
obtained a bright yellow solution. This suggests iron chloride I believe. |
Most likely a neodymium sulphate. Many of us have noticed there are several forms of Nd sulphate hydrate.
To check, isolate precipitate and treat it with strong NH3 and some time. Wash carefully and dissolve precipitate in HCl (NOT sulphuric),
then test solution with oxalic acid for Nd. I'd put good money on a positive Nd test, executed that way.
[Edited on 12-7-2016 by blogfast25]
|
|
|
| Pages:
1
..
19
20
21
22
23
24 |