Reference
Anders Hoveland

Posts: 13
Registered: 12-7-2010
Member Is Offline
Mood: No Mood
|
|
H+Sponge/easy12-ring-resonance synthesis idea
I had an idea to use simple starting molecules that might self-arrange themselves into a 12-membered ring with delocalized electrons, analogous to
benzene. This ring might be stabilized by the strong coordination of a hydrogen ion in the center, similar to 1,8-Bis(dimethylamino)naphthalene, well
known as "proton sponge" for its strong coordination.
Because of the resonance ring, and the strong bonding to the central H+ the resulting molecule probably would have high stability, so that entropy
would favor its creation. An example of such a favored arrangement is the formation of guanidine from a molten mix of sulfamic acid and urea. The
guanidine ion (CN3H6+) is so stable that it drives the reaction.
In the picture, the positive charge resonates among three nitrogen atoms. Guanidine, as well, has 3 nitrogen atoms to distribute a positive charge
from a hydrogen ion, and guanidine is a strong base.
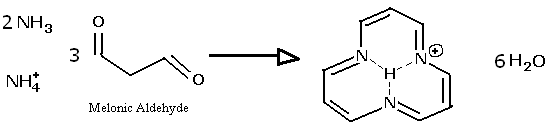
If anyone has the ability to get their hands on melonic aldehyde, which has the structure O=CH-CH2-CH=O ,
you could attempt to make the ring described here. Because of the resonance, the reaction solution might become colored, even if the ring is only in
equilibrium. For example, carotene and diazo-dyes invlove unsaturated and delocalized bonding. If no color is observed, the addition of copper might
create colors not seen in a solution of copper and ammonia alone, since copper forms a wide and heterogeneous series of colored coordination complexes
with organic amines. Several bio-pigments, such as chlorophyll, have large ring molecular resonances, and many are in addition able to bind metal ions
at the center, which changes the frequencies absorbed.
NH=CH-CH2-CH=O should have a tautomer:
NH2-CH=CH-CH=O which could potentially condense into a trimer ring
Some References:
Melonic aldehyde is mentioned in: Schaum's outline of theory and problems of organic chemistry p200
By Herbert Meislich, George J. Hademenos
Information on proton sponge: http://en.wikipedia.org/wiki/1,8-Bis%28dimethylamino%29napht...
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
According to Hückel's rule your compound cannot be benzene-like aromatic molecule and it remains strained even if protonated.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
To be aromatic, with significant electron delocalization and bond orders all of 1½ derived from conjugated alternate double and single bonds, it
would have to have either 2 (cyclopropenyl cation), 6 (benzene, multi-benzenoid rings such as naphthalene etc., azulene, tropylium cation,
cyclopentadiene anion, cyclooctatetraenyl dication), 10 (cyclooctatetraenyl dianion, 2,4,6,8,10-cyclodecapentaene), 14, or 18 etc., pairs of electrons
in pi orbitals in a planar ring, each pair above and below the plane of the ring. Some of the C atoms can be replaced by compatible, often
charge-bearing, heteroatoms such as N, P, S, O, B. In the cases of rings with 10 or more carbons (or compatible heteroatoms), to achieve planarity
some of the bonds usually have to be trans rather than cis.
So that 12-membered ring above, although it may be nearly planar due to the trans-bonds, cannot be aromatic.
|
|
|
|