| Pages:
1
2 |
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
Diphenhydramine hydrochloride
I just want to share my studies and research synthesis ofdiphenhydramine HCl the first generation antihistamines and is often used as a
non-prescription sleep aid and a mild anxiolytic in for example APAP, others info on wiki so he we go.
First step is synthesis of benzhydrol we make it from benzophenone and reduce it with NaBH4 in methanol (0,025 mole benzophenone , 0,026 mole NaBH4
and 50 ml methanol).
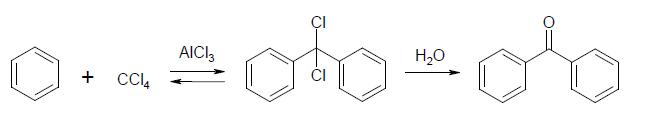
If someone don't know how to do benzphenone here it is synthesis:
In a dry 500 cc. flask put 25 grams of benzene, 25 grams of benzoyl chloride, 50 cc. of carbon bisuiphide, and 30 grams of anhydrous aluminium
chloride. The aluminium chloride should not be exposed to the air any longer than necessary, as it rapidly absorbs moisture. Connect the flask with a
reflux condenser and heat it on a water-bath. The reaction is complete when a few drops of the solution in the flask, when treated with a little
water, give oil which does not have the disagreeable odor of benzoyl chloride. The time required is from 3 to 4 hours. Cool the flask in water, and
pour the contents into about 300 cc. of water to which has been added 20 cc. of concentrated hydrochloric acid. Separate the layer of carbon
bisulphide in which the benzophenone is dissolved, and evaporate off the solvent on the steam-bath in the hood, away from any flames. If no steam-bath
is available, place the solution in a flask and distil off the solvent on a water-bath through a long condenser. Add 50 cc. of alcohol and 2 grams of
bone-black, boil for about 5 minutes, and filter hot. Wash the bone-black with a few cubic centimeters of hot alcohol. When the filtrate is cold, add
cold water until a slight permanent cloud is produced, and set aside to crystallize. If an oil separates, scratch the inside of the beaker just below
the surface of the solution with a glass rod. At the end of some hours or at the next exercise, filter off the crystals by suction and wash them twice
(§12, page 7) with a mixture, of 2 volumes of alcohol and 1 of water. A further amount of benzophenone can be obtained by adding to the filtrate from
the crystals cold water until the solution clouds, and proceeding as above.
Weigh the product and calculate the percentage yield from the benzoyl chloride used. Write equations for all the reactions involved, including those
into which the aluminium chloride enters.
Benzophenone melts at 48°, and boils at 306.1° (corr.). The yield in the preparation is about 20 grams.
So now we have benzhydrol and the next step is to make our diphenhydramine hydrochloride.
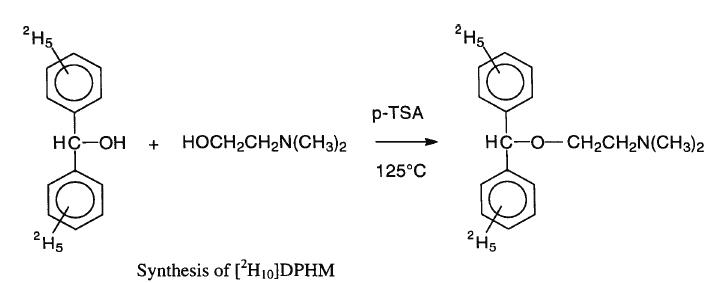
Into a clean three neck 250 ml round bottom flask 2,5 g purified benzhydrol , 1,5 dimethylaminoethanol, 2,7g para-toluene sulfonic acid, 27 ml
tetrachloroethane and 41 ml toluene were added. A teflon coated magnetic stirrer,a reflux condenser, a thermometer and a water trap were added into
the reaction flask. The reaction was conducted under a nitrogen atmosphere. The reaction was initiated by gradually heating the reaction mixture to
125 C with stirring. The reaction mixture was refluxed for 48 hours, cooled to 60 C and diluted with 90 ml petroleum ether and 30 ml disteilled water.
The reaction mixture was transferred to 50 ml separatory tunnel. The bottom two aqueous layers were removed and transferred to a celan seperatory
funnel. The top organic phase was washed with two 20 ml aliquots distilled water and the washings were combined with the aqueous fraction. A 7 ml of
50% aqua NaOH was added to the aqueous phase. Next, the aqueous phase was extracted with one 60 ml and two 30 ml portions of diethyl ether. The
combined ether phase was washed with two 20 ml portions of water and then dried twice with 4g portions of anhydrous magnesium sulfate. This mixture
was filteres and the ether removed under vacuum. A 2 ml aliquot isopropyl alcohol was added to the resulting oil, followed by addition of 2,6 ml
isopropyl alcohol saturated with HCl (HCl was bubbled throught the isopropanol). This followed by addition of 70 ml anhydrous diethyl ether. The
mixture was allowed to stand at 4 C overnight to crystallize. The following morning the mixture was filtered and the resulting crystals dried under
vacuum. The product was re crystallizes twice: once, using 2 ml isopropanol and 60 ml anhydrous diethyl ether, the second time, using acetone and
heat. The yeild was 50%.
In the attachments are reaction to synthesis above chemicals.
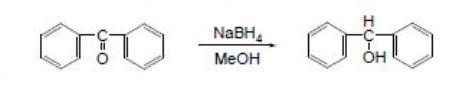
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
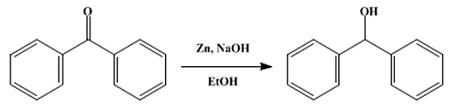
I just want to add : for making benzohydrol you need for someone hard to obtain NaBH4 , but i found another simple method of reduce it with only
Zn/HCl :
In a 3-l. round-bottomed flask fitted with a mechanical stirrer are placed 200 g. of technical flake sodium hydroxide, 200 g. of benzophenone, 2 l. of
95 per cent alcohol, and 200 g. of technical zinc dust. The stirrer is started and the mixture slowly warms to about 70° spontaneously. After two to
three hours the mixture, which has started to cool, is filtered with suction, and the residue is washed twice with 100-cc. portions of hot alcohol.
The filtrate is poured into five volumes of ice water acidified with about 425 cc. of concentrated commercial hydrochloric acid. Thebenzohydrol
separates as a white crystalline mass and is filtered by suction. The yield of crude air-dried product melting at 65° is 194–196 g. (96–97 per
cent of the theoretical amount). From 200 g. of crude product in 200 cc. of hot alcohol there is obtained, after cooling in an ice-salt mixture,
filtering, and drying, 140–145 g. of product melting at 68°. The benzohydrol remaining in the mother liquors may be precipitated with water.
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
How is the ether formed from the two alcohols? Sorry I'm such a noob, I haven't seen that reaction before. Surely you don't mix the reagents all at
once, unless the p-TSA only reacts with one of them.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I have heard of ether formation from two alcohols however i cant remember the reagent which performs such, I didnt know P-TSA could do it.
I myself am very interested in making DPH however i have found it impossibple to obtain/make the diethylaminethanol.
Benzophenone can be reduced with aluminium isopropoxide with very good yields (speaking from experience) and the compound does not need to be
purified, the crude produce form aluminium + isopropyl alcohol works fine.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Mario840, thanks for sharing, but you did not explain if this is something you were doing from some published example, your modification of a
published synthesis, or something new. I'm sure I'm not the only one wandering about it. In any case your posts lacks the pertaining references.
Please add them so that we have enough information for a proper discussion.
| Quote: | | Into a clean three neck 250 ml round bottom flask 2,5 g purified benzhydrol , 1,5 dimethylaminoethanol, 2,7g para-toluene sulfonic acid, 27 ml
tetrachloroethane and 41 ml toluene were added. |
Is that 1.5g N,N-dimethylaminoethanol? That would mean there is an 18% excess of the amine over the TsOH×H2O (or 7% excess if you used anhydrous
TsOH). Usually a few mol% of TsOH is used in SN1 substitutions on benzhydryl alcohol, while here you have only the ammonium tosylate as the acid
catalyst and furthermore a slight excess of the free amine. Seems counterintuitive. Is there are an experience based reason for this? Have you tried
with an actual excess of TsOH and what were the results?
Also, I assume you used tetrachloroethene and the "tetrachloroethane" is only a typo.
Quote: Originally posted by Picric-A  | | I have heard of ether formation from two alcohols however i cant remember the reagent which performs such, I didnt know P-TSA could do it.
|
It is an SN1 substitution quite commonly used for forming benzhydryl ethers (Ph2CH-O-R). In fact it is the only way, because SN2 substitutions don't
work, neither for the alkylation of Ph2CH-OH or alkylation with Ph2CH-X (steric reasons). Even benzhyryl halides can be used in SN1 reactions under
basic conditions (there are no beta-hydrogens in Ph2CH-X so no elimination), but this is more or less limited to solvolysis reactions due to practical
reasons (for example for the formation of Ph2CH-O-R where R comes from the alcohol used as the solvent ROH). Some of this was already discussed in an earlier thread.
Another interesting (mechanistically) synthesis of this compound is the rearrangement (actually a self alkylation) of the quaternary ammonium salt:
Ph<sub>2</sub>CH-N<sup>+</sup>Me<sub>2</sub>-CH<sub>2</sub>CH<sub>2</sub>OH =>
Ph<sub>2</sub>CH-O-CH<sub>2</sub>CH<sub>2</sub>N<sup>+</sup>Me<sub>2</sub>H (as chlorides)
Helvetica Chimica Acta, 38 (1955) 1712-1720.
Apparently this is what occurs in a direct synthesis by heating benzhydryl bromide, N,N-dimethylethanol and K2CO3 as described in Zhurnal Obshchei
Khimii, 21, (1951) 570-574 (any other explanation would not make sense).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
Thank you for reply gentlemen i'm glad and i want to explore this thread.
I made this synthesis .... 1,5 g of dimethylethanolamine (yes it is N,N-dimethylaminoethanol you just write diffrent  it sould be N,N-Dimethylethanolamine also known DMAE or 2-Dimethylaminoethanol ,
C4H11NO) it sould be N,N-Dimethylethanolamine also known DMAE or 2-Dimethylaminoethanol ,
C4H11NO)
I used para-toluene sulfonic acid * hydrate , here it is: C7H6O3S*H2O
Yes I used tetrachloroethane (1,1,2,2-Tetrachloroethane) sorry for that mistake.
Reaction start when you heat 125 - 130 C.
Now i'm really focused on synthesis of N,N-Dimethylethanolamine , my friend gave me about 100 ml (it's really cheap , he got this from across , i
checked 1 liter = 27,2 euro) but i love chemistry and i want to make it my own.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You can prepare the target compound also without dimethylethanolamine, but it takes more steps. Chloroethanol can be alkylated with benzhydryl alcohol
to give benzhydryl 2-chloroethyl ether (see Organic Syntheses) to be used in the alkylation of dimethylamine. Alternatively, ethylene glycol can be alkylated with benzhydryl alcohol to give
benzhydryl 2-hydroxyethyl ether (see p23, supporting material of JACS, 131 (2009) 1766–1774), which can be transformed to a tosylate ester (see Monatshefte fuer Chemie, 89 (1958) 342-345) and this used to alklyate dimethylamine.
I would appreciate if you would answer to the questions above.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
I made the experiment based on published synthesis , here You have : https://circle.ubc.ca/bitstream/handle/2429/8809/ubc_1995-98...
I try to "cut" amount of N,N-dimethylaminoethanol but the yield was lower , the amount of benzhydrol may be higher even 2,7 - 3 g , yield: 55% , the
para-toluene sulfonic acid act like catalyst , next time i will change amount of this compound , beacuse 50% yield it's not too good for me 
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
And where exactly is your contribution? You copy/pasted a few procedures word by word from an old thesis, gave no reference and presented it as your
work? If you followed that procedure then explicitly say so and describe your experience with it, so to give some sense to the starting of this
thread.
Quote: Originally posted by mario840  |
I just want to share my studies and research synthesis ofdiphenhydramine HCl the first generation antihistamines and is often used as a
non-prescription sleep aid and a mild anxiolytic in for example APAP, others info on wiki so he we go. |
You do understand that coupling this with your last post actually means that you admitted a plagiarism? I may be oldfashioned when it comes to such
issues, but I do consider such behaviour as... well, you can imagine.
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
I research synthesis I found I'll post link above then I studies and experiment how to increase yield , this was my first post so now i link
references , when I will success increase the yield i will post photos and other tips to experminet .... that's all
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Quote: Originally posted by Nicodem  | You can prepare the target compound also without dimethylethanolamine, but it takes more steps. Chloroethanol can be alkylated with benzhydryl alcohol
to give benzhydryl 2-chloroethyl ether (see Organic Syntheses) to be used in the alkylation of dimethylamine. Alternatively, ethylene glycol can be alkylated with benzhydryl alcohol to give
benzhydryl 2-hydroxyethyl ether (see p23, supporting material of JACS, 131 (2009) 1766–1774), which can be transformed to a tosylate ester (see Monatshefte fuer Chemie, 89 (1958) 342-345) and this used to alklyate dimethylamine.
I would appreciate if you would answer to the questions above. |
Nicodem- would the alkylation of benzhydryl with ethylene glycol use the same conditions and the alkylation with dimethylaminoethanol? (ie, p-TSA)
Are there any more readily available substitutes for the p-TSA in this reaction?
If i obtain the benzhydryl 2-hydroxyethyl ether in good yield i would probably proceed by reaction with SOCl2 to produce the alkyl chloride then
react this with dimethylamine with triethylamine present to remove the HCl formed, leaving me with diphenhydramine.
Would this work?
Thanks,
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Picric-A  | | Nicodem- would the alkylation of benzhydryl with ethylene glycol use the same conditions and the alkylation with dimethylaminoethanol? (ie, p-TSA)
|
Conditions required for the SN1 substitutions suitable for this specific combination of reactants:
Yes. Sulfuric acid for example:
Quote: Originally posted by Picric-A  | If i obtain the benzhydryl 2-hydroxyethyl ether in good yield i would probably proceed by reaction with SOCl2 to produce the alkyl chloride then
react this with dimethylamine with triethylamine present to remove the HCl formed, leaving me with diphenhydramine.
Would this work? |
It is unlikely. SOCl2 is generally not a benign reagent for sensitive ethers like benzyl or benzhydryl ethers or any other such one that is sensitive
to acids. However there are alternatives:
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Quote: Originally posted by Picric-A  | If i obtain the benzhydryl 2-hydroxyethyl ether in good yield i would probably proceed by reaction with SOCl2 to produce the alkyl chloride then
react this with dimethylamine with triethylamine present to remove the HCl formed, leaving me with diphenhydramine.
Would this work?[/rquote]
It is unlikely. SOCl2 is generally not a benign reagent for sensitive ethers like benzyl or benzhydryl ethers or any other such one that is sensitive
to acids. However there are alternatives:
|
Could you possibly copy/paste the relevant part of this article as i do not have access to it.
So instead i will react benzhydryl with ethylene glycol in methylbenzene with conc H2SO4 as the catalyst. This should give me the benzhydryl
2-hydroxyethyl ether.
Edit: Will the Mitsunobu Rxn work the this particular substrate?
It may give better yields than mario says he got (50%) and DEAD is fairly simple to make if once has access to hydrazine.
[Edited on 29-7-2010 by Picric-A]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I don't feel like going to the library and waste a few hours just to do you a favour. Try in the References section like others do.
| Quote: | | So instead i will react benzhydryl with ethylene glycol in methylbenzene with conc H2SO4 as the catalyst. This should give me the benzhydryl
2-hydroxyethyl ether. |
Hmm... for someone who only a couple of days ago never even heard about SN1 nucleophilic substitutions on benzhydryl alcohols, you became quite over
ambitious. How about explaining what role would toluene have and why do you think having a biphasic toluene/ethylene glycol system is going to help (I
don't think these two solvents are miscible). I can only see negative aspects: temperature limited by the toluene reflux, benzhydryl alcohol
preferentially partitioning in the toluene phase and H2SO4 in the glycol phase, etc.
Maybe you could first explain what was it that you found impractical in the procedure from JACS? I really can't see how more simple you could have
it. If you don't have any tosylic acid, then try with a similar equivalent of NaHSO4, H2SO4, H3PO4 or any other non-nucleophilic strong enough acid
and follow with TLC (the reaction rate will change if you use a different acid catalyst!). If the reaction rate gets too slow due to the catalyst
exchange, then add some more or heat up by 20°C or so.
| Quote: | Edit: Will the Mitsunobu Rxn work the this particular substrate?
It may give better yields than mario says he got (50%) and DEAD is fairly simple to make if once has access to hydrazine. |
The Mitsunobo reaction is for nucleophiles that form in situ via deprotonation of a compound which needs to be relatively acidic (pKa must be up to 11
or thereabout : carboxylic acids, HN3, imides, phenols, etc.). Thus it does not work on amines as nucleophiles, at least as far as I know (see the
mechanism to understand why).
And say that preparing DEAD is fairly simple only once you will prepare some.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Quote: Originally posted by Nicodem  |
| Quote: | | So instead i will react benzhydryl with ethylene glycol in methylbenzene with conc H2SO4 as the catalyst. This should give me the benzhydryl
2-hydroxyethyl ether. |
Hmm... for someone who only a couple of days ago never even heard about SN1 nucleophilic substitutions on benzhydryl alcohols, you became quite over
ambitious. How about explaining what role would toluene have and why do you think having a biphasic toluene/ethylene glycol system is going to help (I
don't think these two solvents are miscible). I can only see negative aspects: temperature limited by the toluene reflux, benzhydryl alcohol
preferentially partitioning in the toluene phase and H2SO4 in the glycol phase, etc.
Maybe you could first explain what was it that you found impractical in the procedure from JACS? I really can't see how more simple you could have
it. If you don't have any tosylic acid, then try with a similar equivalent of NaHSO4, H2SO4, H3PO4 or any other non-nucleophilic strong enough acid
and follow with TLC (the reaction rate will change if you use a different acid catalyst!). If the reaction rate gets too slow due to the catalyst
exchange, then add some more or heat up by 20°C or so.
|
I was simply substituting bezene for toluene as they have very similar chemical properties other than the fact toluene is much less dangerous. Benzene
is used in the Org Syn write-up and so i assumed this would be fine. Benzene, like toluene is immicible with ethylene glycol and so i guessed the
biphasic system worked! Im not trying to say im excellent, far from it, im just trying to adapt published articles.
Like i said i dont have access to the article from JACS and i dont have any tosylic acid. If you could highlight main ideas form the article mentioned
that would be great, exact copy isnt needed.
Thanks
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Slightly off-topic: TsOH is in no way an insurmountable difficulty for home chemists as it can easily be made from toluene and sulfuric acid (search
the net). Unless multiple recrystallizations are performed, product is red instead of white, but it doesn't seem to matter a lot, since it was
successfully applied for the synthesis of a certain Schiff base. For some reason even when azeotropically drying commercial TsOH hydrate with toluene
on a Dean Stark trap a very dark red solution is obtained. No idea why.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Picric-A  | | I was simply substituting bezene for toluene as they have very similar chemical properties other than the fact toluene is much less dangerous. Benzene
is used in the Org Syn write-up and so i assumed this would be fine. Benzene, like toluene is immicible with ethylene glycol and so i guessed the
biphasic system worked! Im not trying to say im excellent, far from it, im just trying to adapt published articles. |
Huh? You mixed up everything. The Org. Synth. article is about a reaction between benzhydryl alcohol and 2-chloroethanol to give a different product.
2-Chloroethanol is something different from ethylene glycol and furthermore miscible in benzene or toluene. The synthesis of benzhydryl 2-hydroxyethyl
ether is described in the supporting info of the JACS paper! As well as in a few other papers, but with more or less similar procedures.
| Quote: | | Like i said i dont have access to the article from JACS and i dont have any tosylic acid. |
Previously you said you did not have access to the Monatshefte paper, while everybody that has access to the internet has access also to the
supporting material at ACS. Sorry, but I simply do not believe you that you can't get that page 23. Open the first document in the supporting info
section and get to its page 23. You need a PDF viewer installed on your PC.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Quote: Originally posted by Nicodem  | Quote: Originally posted by Picric-A  | | I was simply substituting bezene for toluene as they have very similar chemical properties other than the fact toluene is much less dangerous. Benzene
is used in the Org Syn write-up and so i assumed this would be fine. Benzene, like toluene is immicible with ethylene glycol and so i guessed the
biphasic system worked! Im not trying to say im excellent, far from it, im just trying to adapt published articles. |
Huh? You mixed up everything. The Org. Synth. article is about a reaction between benzhydryl alcohol and 2-chloroethanol to give a different product.
2-Chloroethanol is something different from ethylene glycol and furthermore miscible in benzene or toluene. The synthesis of benzhydryl 2-hydroxyethyl
ether is described in the supporting info of the JACS paper! As well as in a few other papers, but with more or less similar procedures.
| Quote: | | Like i said i dont have access to the article from JACS and i dont have any tosylic acid. |
Previously you said you did not have access to the Monatshefte paper, while everybody that has access to the internet has access also to the
supporting material at ACS. Sorry, but I simply do not believe you that you can't get that page 23. Open the first document in the supporting info
section and get to its page 23. You need a PDF viewer installed on your PC. |
I am very sorry for my stupidity here Nicodem, i can see the article i was just clicking on the wrong link (to buy the article)
Your right this article is exactly what i need and is very detailed. Will it be fine for my to substitute the p-TSA here for H2SO4?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Picric-A  | I am very sorry for my stupidity here Nicodem, i can see the article i was just clicking on the wrong link (to buy the article)
Your right this article is exactly what i need and is very detailed. Will it be fine for my to substitute the p-TSA here for H2SO4?
|
And here die all my hopes that any one actually reads my replies...
Quote: Originally posted by Nicodem  | Maybe you could first explain what was it that you found impractical in the procedure from JACS? I really can't see how more simple you could have
it. If you don't have any tosylic acid, then try with a similar equivalent of NaHSO4, H2SO4, H3PO4 or any other non-nucleophilic strong enough acid
and follow with TLC (the reaction rate will change if you use a different acid catalyst!). If the reaction rate gets too slow due to the catalyst
exchange, then add some more or heat up by 20°C or so.
|
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Quote: Originally posted by Nicodem  | Quote: Originally posted by Picric-A  | I am very sorry for my stupidity here Nicodem, i can see the article i was just clicking on the wrong link (to buy the article)
Your right this article is exactly what i need and is very detailed. Will it be fine for my to substitute the p-TSA here for H2SO4?
|
And here die all my hopes that any one actually reads my replies...
Quote: Originally posted by Nicodem  | Maybe you could first explain what was it that you found impractical in the procedure from JACS? I really can't see how more simple you could have
it. If you don't have any tosylic acid, then try with a similar equivalent of NaHSO4, H2SO4, H3PO4 or any other non-nucleophilic strong enough acid
and follow with TLC (the reaction rate will change if you use a different acid catalyst!). If the reaction rate gets too slow due to the catalyst
exchange, then add some more or heat up by 20°C or so.
|
|
Reads/takes in are two completly different ideas.
I will try this reaction asap with different catalysts and see which yields greater yields.
Thanks for all your help.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I performed the reaction today with a H2SO4 catalyst and i got a 62% yield, im not sure how i can improve the yield however because i followed the
follow up exactly (apart from the cat.)
Now i need to add diethylamine to the benzhydryl 2-hydroxyethyl ether. Now normally i would add a chloride group to act as the leaving group however
Nicodem said this does not work does as thionyl chloride would cleave the ether group.
Is there another method of adding on the dimethylamine? Preferably using easily sourced reagents.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Picric-A  | | I performed the reaction today with a H2SO4 catalyst and i got a 62% yield, im not sure how i can improve the yield however because i followed the
follow up exactly (apart from the cat.) |
What is the melting point of your product?
| Quote: | | Now i need to add diethylamine to the benzhydryl 2-hydroxyethyl ether. Now normally i would add a chloride group to act as the leaving group however
Nicodem said this does not work does as thionyl chloride would cleave the ether group. |
I said it is unlikely, which means that it might work but that I'm more convinced that it would not rather than would. I could be wrong. Besides, it
very much depends on the conditions used. Perhaps if you use a large excess of pyridine or do the reaction in DMF instead of CH2Cl2... You should
check the literature for similar examples.
| Quote: | | Is there another method of adding on the dimethylamine? Preferably using easily sourced reagents. |
Synthesis of ethylsulfonyl chloride
Dichlorocarbene chlorination of alcohols in an alkaline micelle.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I got a m.p. of 127degC, however i can not find any literature stating the actual m.p of the compound.
I might give the thionyl a shot then do a TLC to find out whether it cleaved the ether or not... i guess its worth a shot. I dont have any DMF so i
guess CH2Cl2 or CHCl3 are my only options.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I sincerely can not think of anything that could result from this reaction and have that mp. The desired product should have the mp of 60-70°C
(different sources give different data). Benzhydryl alcohol has a mp of 65-68°C, so it would be hard to determine the identity of the product solely
by mp, but with a confirmation of non-identity from TLC it would be enough. Dibenzhydryl ether has the mp of 104-110°C, so it is not this either.
On your place I would check the identity of your starting materials.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Quote: Originally posted by Nicodem  |
I sincerely can not think of anything that could result from this reaction and have that mp. The desired product should have the mp of 60-70°C
(different sources give different data). Benzhydryl alcohol has a mp of 65-68°C, so it would be hard to determine the identity of the product solely
by mp, but with a confirmation of non-identity from TLC it would be enough. Dibenzhydryl ether has the mp of 104-110°C, so it is not this either.
On your place I would check the identity of your starting materials. |
Wow that is indeed far off... I guess dibenzhydryl ether seems the most likely product... maybe with other impurities which lead to to the raised B.P
?
I will run a TLC to see what impurities are in my product, i dont have any pure compound though for comparison.
|
|
|
| Pages:
1
2 |