| Pages:
1
2 |
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
Perfluorooctanesulfonic acid (PFOS) from KPFOS
I need to prepare PFOS:
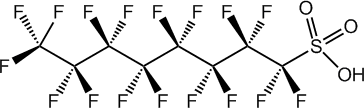
From it's potassium salt. The problem is there doesn't seem to be much useful information on the internet or in the literature on it's
chemical/physical properties. I have attempted adding a few drops of conc. H2SO4 to KPFOS "dissolved" (extremely low solubility IN FREAKING
EVERYTHING) in acetonitrile. A free-flowing solid formed at the bottom (replacing the extremely hydrophobic layer that was once there; it looked like
Squand underwater). I should've tested its solubility in water to see if it was K2SO4 but I haven't yet.
Also, is PFOS a liquid or solid? Wikipedia gives its boiling point: 133*C (at 6 torr), but that's it. I can only imagine that, with such a
high boiling point at such a low pressure, it must be a solid. But I can't find anything to confirm or deny this.
Alternatively, (this is better but much more difficult) I need to prepare a salt of PFOS that is soluble in acetonitrile, propylene carbonate or even
water. Some have reported tetraethylammonium PFOS (TEAPFOS) to be soluble in acetonitrile (I'll grab the reference if anyone needs it). Now, how
could I synthesize this from it's potassium salt? I have compounds with a tetrabutylammonium cation but no TEA (that I know of).
Any ideas? Thanks in advance!
EDIT:
According to this article, the solubility of PFOS in acetonitrile is at least 0.12 M.
[Edited on 8-3-2010 by Mister Junk Pile]
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Well, the first thing you should have done is check whether or not acetonitrile reacts with sulphuric acid. It does, violently if you are unlucky.
|
|
|
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
It did not seem to react appreciably. A few bubbles maybe but I thought these might be just trapped air from shaking.
No heat either, that I could tell.
I will try it in water. Will that be acceptable? Will it work? Basically: will the PFOS react with H2SO4? Or other acids for that matter? I
really don't see it being very reactive at all. What do you say?
[Edited on 8-3-2010 by Mister Junk Pile]
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
I think you might find something useful in these references:
1) Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications
http://ifile.it/xkj3ezv/36860___mfoc.rar
2) Handbook of Fluorous Chemistry
http://ifile.it/3gcwes/wsedrf.rar
|
|
|
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
Thank you for the references matei. I will enjoy reading them (although I only have until Friday to figure this out).
I realize this isn't as interesting as the synthesis of fentanyl precursors or DMT but, does anyone else have any ideas or experience that they can
share? I will be waiting patiently and hoping for some awesome replies.
Thanks.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Try partitioning your potassium salt between H2SO4 and petroleum ether. Separate the petroleum ether extract and rotavap. Distil under vacuum if
necessary. If this does not work you can always try vacuum distilling it from a mixture of potassium perfluorooctanesulfonate with some nonvolatile
acid: NaHSO4, H3PO4 or one equivalent of H2SO4.
As for the tetraethylammonium salt, try with a suspension of potassium perfluorooctanesulfonate and Et4NCl in acetonitrile (with a slight excess of
the sulfonate) stirring for a day or two. Then filter off the insolubles, rotavap the filtrate and recrystallize from whatever suitable solvent. Mind
to use enough acetonitrile to dissolve all the formed tetraethylammonium sulfonate, but not too much as KCl is also slightly soluble.
Otherwise the simplest method to prepare tetraethylammonium perfluorooctanesulfonate would obviously be to ethylate triethylamine with ethyl
perfluorooctanesulfonate, but unfortunately ethyl perfluorooctanesulfonate is not commercially available. By the way, you do know that
tetrabutylammonium perfluorooctanesulfonate is commercially available? Obviously, the same goes for the free acid.
|
|
|
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
Yes, I do. But I have limited time and can't wait for shipping.
But wait, Fisher & Aldrich don't seem to have it and I'm not sure I can order from anywhere else.
I found tetrabutylammonium heptadecafluorooctanesulfonate on Aldrich which might work. It's VERY expensive, though, and I don't know if my professor will
authorize $180 for 5 mL (this is a liquid??) of something that might not work. He probably will but, I'd like to try other avenues before I ask him.
I will try your methods of synthesis. I only have a couple of grams left so hopefully this will work. Of course, I won't blame you if it doesn't

|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Apollo, Abcr, Fluorochem, Matrixsci., Oakwood and a bunch of other companies sell the acid. Prices are in the range of 10EUR/g, depending on the
amount.
Quote: Originally posted by Mister Junk Pile  | I will try your methods of synthesis. I only have a couple of grams left so hopefully this will work. Of course, I won't blame you if it doesn't
 |
Even if you would blame me I would not care. Which method do you talk about? If you mean extracting the acid from H2SO4, then mind that you need to
use >80% sulfuric acid, preferably the concentrated one, as the C8F17SO3H is so acidic that it will nearly completely dissociated in any less
concentrated H2SO4 and none would get extracted.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
Yes, that is the one I will try. I will probably use pentane instead of pet. ether. No one seems to have petroleum ether anymore. Also, the
stockroom manager is out so I am stuck with what I have here and down the hall (i.e. no petroleum ether).
I will check elsewhere but I don't see why pentane wouldn't be acceptable, right?
EDIT:
Found some tetraethylammonium bromide. I will try dissolving it in MeCN and adding KPFOS. Do you think 'sonication' would be better than simply
stirring?
[Edited on 8-4-2010 by Mister Junk Pile]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Pentane is a petroleum ether!
|
|
|
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
I understand that but I was always under the impression that petroleum ether was a mixture of pentane, hexane and heptane (depending on various
factors). This, although very similar to neat pentane, is different.
I made some changes to my post above.
I noticed that some people were complaining about people not doing actual chemistry on this website. Well, although most will find what I am about to
do extremely simple and boring, it will give me an excuse to take some pictures. I will probably do it tomorrow, though, as I must leave early today.
Thanks, Nicodem.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
What are perfluorooctanesulfonates and the (strong) acid used for? Without the fluorine atoms substituting for H, these sulfonates would be good
anionic detergents, but the F atoms would greatly reduce the polarity of the anion andf its usefulness as a detergent. Long-chain perfluoroalkanes are
used as lubricants, especially for longer life and ability to stand much higher temperatures than long-chain ordinary hydrocarbons, but addition of
the anionic group would greatly reduce hydrophobicity.
To produce both the fluorocarbon and its sulfonated derivative requires, to begin with, either the Fowler CoF3-mediated process with F2, or the Simons
electrolytic process with HF, to replace the H atoms in hydrocarbons (or derivatives), without rupturing the C-C or other bonds; see http://en.wikipedia.org/wiki/Fluorocarbon , http://en.wikipedia.org/wiki/Perfluorocarbon .
[Edited on 4-8-10 by JohnWW]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I said pentane is a petroleum ether, not that it is petroleum ether. Anyway, the point is in that you use an inert and very nonpolar solvent.
The perfluoroalkyl group is very hydrophobic and even though the sulfonic part is polar, the logP of the non-dissociated acid is relatively high. If
after rotavaping not much is left, then try also extracting with dichloromethane.
I would not be so sure KBr is enough insoluble in acetonitrile. I would check its solubility before using Et4NBr. If you would at least have some more
info about tetraethylammonium perfluorooctanesulfonate solubility in other solvents you could use some better choice of a solvent. Something like
ethyl acetate or toluene would be perfect if the quat sulfonate would be soluble in it. KBr is practically insoluble in ethyl acetate, toluene,
dichloromethane, chloroform and other such solvents.
Yes, sonication would be even better than stirring, so use that if your coworkers don't mind the acoustic annoyance. You can also heat it up. It might
be possible to check the progress by doing IR readings of the solution (without the insolubles!). Just put a drop on an NaCl window for IR, dry it and
run an IR measurment. Identify the changing IR peaks and continue the metathesis until the IR spectra does not change any more. From your first post
it seems to me that you work in an electrochemical lab, so I can also suggest you to follow the metathesis conductometricaly.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
Thank you. Yes, I work in what could be called an electrochemical lab. I am simply an undergraduate though. I don't have an IR spectrophotometer
but other labs down the hall do (which is also where the sonicator is located).
I am leaving now. Tomorrow is a new day. Thanks for all the help/info.
[Edited on 8-4-2010 by Mister Junk Pile]
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
The second method Nicodem suggested was actually used to get PFOS out of its K salt, according to J. Chem. Soc., 1957, 2640
(see attachment). All you have to do is vacuum distil the acid (PFOS) from a mixture of potassium salt and concentrated (98%) sulfuric acid. The
boiling point of PFOS is 145 deg. C at 10 torr. I don't know what is the boiling point of sulfuric acid at that pressure, maybe you'll have to use a
fractionating column. Metal salts of PFOS are apparently made by neutralizing the acid with a metal hydroxide, however I don't know what solvent, if
any, is used for this (see also J. Phys. Chem., 1972, 76, 909).
The solubility of the tetraethylammonium salt of PFOS in various salts, and possibly its preparation can be found in Tenside,
1978, 15, 2 (this is a German journal not available online).
Check also this book, which is a little more on the subject:
Fluorinated Surfactants and Repellents, 2nd Ed., (pass: ebooksclub.org)
http://ifile.it/1cs8fpl/sUBl9gYSo.7z
Attachment: Perfluoroalkyl derivatives of sulphur. Part VI. Perfluoroalkanesulphonic acids.pdf (151kB)
This file has been downloaded 924 times
|
|
|
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
Theory and Procedure
There is a paper (I'm not at my computer right now but I will post it later) that tells of creating a superhydrophobic polypyrrole film via the
electropolymerization of pyrrole in acetonitrile with TEAPFOS and a catalytic amount of FeCl3. Somehow, due to the combination of electro and
catalytic polymerization, the surface becomes "rough" at the micro scale and thus has a water contact angle of greater than 150 degrees (see Wikipedia
for this if you are not familiar).
Apparently the PFOS anion is "attracted" to the delocalized/distributed positive charge on the pyrrole backbone (due to protonation of the -NH- group)
therefore exposing whatever substance is applied to the surface to the extremely electronegative and hydrophobic 'tail' of the surfactant. This, of
course, only helps the one of the two elements of hydrophobicity (morphology and chemical properties).
This is how I understand it. Most mechanisms I have seen for the electropolymerization of pyrrole involve forming a pyrrole radical and then forming
the chain from there. I have a paper on this as well if you wish to see it.
There are other methods that involve placing fluoroalkyl substituents on the pyrrole moeity but I do not have the materials to attempt something like
that at the moment.
Also, I probably shouldn't talk about any modifications to the process until I'm finished, right? How does that work?
QUICK UPDATE:
I created a sat'd solution of TEABr in DCM (precipitate on bottom even after an hour or so) and weighed a glass petri-dish + lid. Then, I took 2 mL
of this solution, boiled off the DCM and then dried the crystals in a vacuum oven (10 torr or so) at 110*C for 30 mins. No sublimation was observed.
Crystals remaining were 1.2562 g which gives a solubility of 0.6281 g/mL (62.81 g/100 mL). I know this isn't TEAPFOS, but at least I know that TEABr
is very soluble in DCM. I will try the displacement in DCM.
Procedure:
In preparation, both the PFOS and TEABr were dried in a vacuum oven at 110*C for one hour. The dichloromethane was dried over CaCl2 (placed in a test
tube and the CaCl2 added) for an hour with frequent vortexing. 0.258(6) g of TEABr (F.W. = 210.16) was weighed, placed in the test tube and then
vortexed until completely dissolved. Then, an excess of KPFOS (F.W. = 538.22) was weighed and then placed into the test tube (0.7012 g). This
mixture was sonicated for one hour.
A fine free-flowing precipitate was observed at the bottom of the test tube. Left to sit, will come back and start again tomorrow.
[Edited on 8-5-2010 by Mister Junk Pile]
|
|
|
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
The liquid was decanted and dried in a vacuum oven at 100*C. The white powder was dissolved in DCM once more and gravity filtered. The DCM was
evaporated under vacuum. Yield was 0.4298 g (55.5%). The yield is low because while drying in the oven the solution began bubbling/foaming violently
at 15 in. Hg and some spilled out of the beaker.
The crystals were soluble in water, DCM, acetonitrile and propylene carbonate. I have not tested melting point yet. I will get to it. The
precipitate could not be melted even on a hot plate turned up all the way which leads me to believe that it is, indeed, KBr.
Is there anything else I should do?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mister Junk Pile  | | The liquid was decanted and dried in a vacuum oven at 100*C. The white powder was dissolved in DCM once more and gravity filtered. The DCM was
evaporated under vacuum. Yield was 0.4298 g (55.5%). The yield is low because while drying in the oven the solution began bubbling/foaming violently
at 15 in. Hg and some spilled out of the beaker. |
You know, evaporations are done with a rotavapor and not vacuum ovens at 100°C.
Interesting that the solubility in CH2Cl2 is so good. Not that I'm surprised, but this means you could do the metathesis more efficiently in this
solvent instead of acetonitrile which tends to dissolve salts, such as potassium's, much more.
| Quote: | The crystals were soluble in water, DCM, acetonitrile and propylene carbonate. I have not tested melting point yet. I will get to it. The
precipitate could not be melted even on a hot plate turned up all the way which leads me to believe that it is, indeed, KBr.
Is there anything else I should do? |
A mp is enough if it is known, though for a compound with a perfluorooctyl chain the mp interval might be wide and this can be misleading in regards
to deducing purity. For publications you need also at least an IR (which however would not tell you much). Unfortunately an 1H NMR spectra would not
tell much either given only the Et4N<sup>+</sup> would show up. But a combination of 1H, 13C and 19F NMR spectra would tell the identity,
though again not purity. The proper way to check the purity would therefore by the CHN elemental analysis. (Edit: Forget the 13C NMR. It would be
useless mess of multiplets. For a moment I forgot about the annoying C-F couplings.)
[Edited on 7/8/2010 by Nicodem]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | (Edit: Forget the 13C NMR. It would be useless mess of multiplets. For a moment I forgot about the annoying C-F couplings.) |
Shouldn't a decent NMR operator be able to record broadband F decoupled spectra? Especially in this case, where proton decoupling isn't even
necessary?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I don't know. I never even recorded a normal 19F spectra let alone a 13C decoupled from 19F, but in principle it should be possible to decouple
19F-13C just like it is done with 1H-13C in normal 13C NMR. As far as I know it should even be possible to have a double decoupling, so it should be
possible to actually have a normal looking 13C spectra of this salt if the 19F and 1H nucleus are kept saturated. But I have no idea how is this done
in practice and what kind of a probe is required (for decoupling 19F-13C a normal QNP probe should do, or so I think, but for a double decoupling I
truly have no idea).
But all this is, beside being an interesting curiosity, obsolete and would be a total overkill for this specific case, because there is no structural
problem requiring a 13C spectra. And besides integration is useless in 13C NMR so you can not even check if the ions are in 1:1 ratio and since K+ and
Br- don't show up the NMR does not prove much anyway. An MS of the + and - ions would give the same info. In this particular case a CHN analysis tells
more than any NMR and MS analysis, which is unusual as I usually find elemental analysis nearly always a total waste of time in organic synthesis.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
I get an error message - there is a CRC error or somesuch when I try to open the PDF files. Can you upload them again mate? I am VERY interested.
quam temere in nosmet legem sancimus iniquam
|
|
|
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
| Quote: |
You know, evaporations are done with a rotavapor and not vacuum ovens at 100°C.
|
Actually, no I didn't--but now I do know, thanks. My "mentor" was gone for the week. I have never used a rotavapor but it seems like the usage is
pretty straight forward. There is a doctoral student down the hall who has one in his lab. I will use it next time.
I don't know how to use NMR. I didn't go in today for various reasons but I will be taking the melting point tomorrow, if not for anything but to
keep a record. What kind of device is used to take melting points of metals and metal salts? After some searching it seems like this is one way. I don't think we have a diamond-anvil cell here--that would be something to find out.
Anyway, the process was somewhat of a failure. I will do a quick overview right now as I don't have my notebook and I'm tired.
The TEAPFOS and pyrrole were dissolved in propylene carbonate (I will type the exact molarities tomorrow). This was placed in a PTFE electrochemical
reaction vessel. The reference electrode was Ag/Ag+, counter was gold foil and anode was glassy carbon. I electro'd at constant current (~ .25
mA/cm^2 or 1.5 mA) for 1.5 hours. Not much of a film was created so the solution probably needs to be more concentrated. The parts that did form,
though, were most definitely hydrophobic upon inspection with my eyes.
More later...
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mister Junk Pile  |
| Quote: |
You know, evaporations are done with a rotavapor and not vacuum ovens at 100°C.
|
Actually, no I didn't--but now I do know, thanks. My "mentor" was gone for the week. I have never used a rotavapor but it seems like the usage is
pretty straight forward. There is a doctoral student down the hall who has one in his lab. I will use it next time. |
Don't rely solely on your mentor. I rarely saw a mentor worth trusting when it comes to lab techniques or knowledge. The minimum you should do if you
continue working in a lab is to read a few books on lab techniques. Seriously, it is better than asking guidance for every little thing you need to do
and it might even save you from getting injured.
| Quote: | | I don't know how to use NMR. |
You don't have to know before your time. When you would need it, just find someone to do it for you. But if this is not some work aimed for
publication then you don't need any spectroscopic analysis anyway. Also, if it was for publication, your mentor would be very upset to find you
discussing your work on a public forum.
| Quote: | | I didn't go in today for various reasons but I will be taking the melting point tomorrow, if not for anything but to keep a record. What kind of
device is used to take melting points of metals and metal salts? After some searching it seems like this is one way. I don't think we have a diamond-anvil cell here--that would be something to find out. |
At first I thought this was some kind of a joke, but I'm not sure any more. 
http://en.wikipedia.org/wiki/Melting_point_apparatus
Students are still commonly thought to use the Thiele apparatus in their exercises, but nearly nobody uses that any more (except for the home chemists
who have little other choice  ). A microscope with a heating pad is usually used
or at least a slightly more automatized Thiele-like apparatus (Fisher-Johns apparatus). The melting point can also be indirectly obtained from the DSC
analysis. ). A microscope with a heating pad is usually used
or at least a slightly more automatized Thiele-like apparatus (Fisher-Johns apparatus). The melting point can also be indirectly obtained from the DSC
analysis.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Concerning 19F broadband decoupling:
| Quote: | Whereas spin-decoupling in proton NMR and the broadband decoupling of
protons during the acquisition of 13C spectra ({1H}13C) are commonplace, the
decoupling of 19F nuclei is problematic due to the very wide spectral width of
19F spectra. Traditional methods of broadband decoupling (e.g. WALZ, GARP)
do not work well because their limited bandwidths mean that high power levels
need to be employed to cover wide spectral regions.
Recent advances in adiabatic decoupling schemes (e.g. WURST) make it
possible to generate very wide bandwidths with minimum power require-
ments. A recent article [23] describes the experimental considerations ne-
cessary to perform either proton decoupled fluorine ({1H}19F) or fluorine
decoupled proton ({19F}1H) experiments on Varian Inova Spectrometers con-
figured for specialist (e.g. dedicated H�F probe with a third broadband
channel) or general (e.g. AutoSwitchable probe with a single high-band ampli-
fier) use. When the instrument is configured to perform these experiments
the acquisition of 2D 13C�19F correlation experiments is possible. In a sub-
sequent article [105] the implementation of a variety of 19F�1H double
resonance experiments is discussed. Due to the wide variation in proton-
fluorine spin-spin coupling constants, 1H�19F ge-COSY is an experiment
that is ideal for proton-fluorine correlation since no fixed delays, in-
corporated to optimise for a particular value of spin-spin coupling, are re-
quired. 1D 19F filtered HMQC, HMQC-TOCSY and HMQC-NOESY are also de-
scribed. |
Seems like it's not totally trivial and not every machine can do it. But I've found that this is often an advantage, since NMR operators are much more
eager to measure a challenging compound than routine stuff.
Concerning melting points: recrystallize it first to get a nice macro-crystalline sample. Only such a sample will give a reliable melting point (if it
isn't a hydrate/solvate or decomposes or is a solid solution or ...). Inspect it under the microscope (ideally with polarization filters) - this will
often give you a good indication about purity. But nothing conclusive - I've seen cocrystallization of two compounds into perfect crystals and two
polymorphs of the same compound crystallizing in the same recrystallization step. If possible send a few nice crystals to the diffraction lab.
Attachment: 19f.pdf (605kB)
This file has been downloaded 1235 times
|
|
|
Mister Junk Pile
Hazard to Self
 
Posts: 70
Registered: 2-7-2010
Member Is Offline
Mood: No Mood
|
|
| Quote: |
At first I thought this was some kind of a joke, but I'm not sure any more.
|
I know what a melting point apparatus is but I am certain that all of the ones I have used thus far would in no way be able to reach temperatures
needed to melt metal salts (734*C for KBr). The glass (capilary tube) would even begin to soften at that point, right? Allow me to rephrase my
question: what are the differences between a device used to measure the melting point of an organic substance and one used to measure m.p.s of metals
and metal salts? The only device I have ever used is one like this. I thought I deleted that question because I decided to just research it on the internet instead. Oh yeah, I couldn't find anything after a
quick search (except for the diamond-anvil thing) so I gave up and asked (tired).
This might lead to publication but all of the stuff I have been discussing is already in the literature (it's where I got the TEAPFOS idea). Anything
unique has not been mentioned. This was part of a question that I asked earlier: is it usually okay to discuss this as long as I don't divulge
anything unique that is not already out there?
EDIT:
I might not be going back in to work until August 18th, but I will be going in on Thursday, at the latest, to retrieve some notes.
[Edited on 8-10-2010 by Mister Junk Pile]
|
|
|
| Pages:
1
2 |