Random
International Hazard
    
Posts: 1018
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Where to get metals and their carbonates other t?
I am interested in where to get metals, metal oxides, carbonates (or just metal salts that can be turned into other salts of that metal) other than in
pottery and chemical supply store.
I currently have:
Iron - nails
Copper - various things, pipes..
Magnesium - pencil sharpener
Sodium bicarbonate - grocery store
Please post suggestions if you have any.
|
|
|
Gearhead_Shem_Tov
Hazard to Others
  
Posts: 167
Registered: 22-8-2008
Location: Adelaide, South Australia
Member Is Offline
Mood: No Mood
|
|
Assuming you don't mean to prospect for metal ores to smelt, you can just walk around any urban neighbourhood and in the gutter or on the street
you'll find:
• Aluminium
Beverage cans are about 98% aluminium, with 1% manganese and 1% magnesium added for strength and formability. You can also get Al sheet from hardware
stores in the form of roof flashing. And, of course, there's always kitchen foil.
• Chromium
You can sometimes flake off the chrome from rusted out chrome-plated bumpers and the like. This will often be a sandwich of copper, nickel, and
chromium.
• Lead
Wheel balancing weights. These are little sausage-shaped items clip to the car wheel rims, and they are most likely to get knocked off when folks
bump the kerb while parking. I find that if I keep my eyes peeled I can usually find at least one wheel weight for every kilometre I walk on city
streets. These weights were traditionally of lead, or, more recently, zinc alloy, (often 50% zinc & 50% lead, but sometimes all zinc). Either
way, they're easy to melt in a crucible -- or even an empty soup can -- heated by bunsen burner. When you melt them use a stick or wire to brush
away the dross before you pour the molten metal into a mould and to keep the steel clip(s) from tipping out of the crucible.
• Tungsten
Burnt out incandescent lightbulbs will give you small bits of tungsten wire from the filament. Halogen bulbs, the tubular kind, often have much
larger filaments, and their tubes are fused quartz.
• Molybdenum
From incandescents you can also get moybdenum wire; these are the very thin wires supporting the tungsten filament. They usually have one end set
directly into the glass pumping tube and are thus insulated.
• Nickel
The larger wires connecting to to the ends of tungsten filaments are usually nickel between the glass and filament and Dumet wire in the metal/glass
seal. Dumet wire is a copper clad wire with a core of an iron-nickel alloy that matches coefficient of thermal expansion of soda lime glass.
• Zinc
Some wheel weights, as above, but you'll also find zinc in the casing of cheap zinc/carbon battery cells. The squishy black gunk inside is a mixture
of carbon granules, manganese dioxide, and ammonium chloride. The black rod in the centre is a carbon electrode, useful for many things in the lab
once cleaned and brought to a red heat to burn out most of the other chemicals.
• Mercury
Old thermostats have mercury tilt switches in them. Discarded fluorescent light tubes also have a tiny bit of mercury in them, though by the time a
fluorescent tube is thrown in the bin 99% of the mercury will be adsorbed into the phosphors, with the rest amalgamated into the filaments and other
exposed metal bits.
I'm sure other folks could rattle off dozens more examples. You just have to keep your eyes open.
-Bobby
|
|
|
Random
International Hazard
    
Posts: 1018
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Thanks for the information, other please post too if you can add something 
|
|
|
cnidocyte
Hazard to Others
  
Posts: 214
Registered: 7-7-2010
Member Is Offline
Mood: No Mood
|
|
Zinc - The casing of the cells in zinc chloride batteries
Manganese dioxide - Found inside the zinc casing of the cells in zinc chloride batteries
Zinc chloride - Mixed in with the manganese dioxide
Platinum - Some jewellery making shops sell platinum wire
Palladium - ^^
Silver - ^^
Gold - ^^
^^ = same as above
Magnesium from pencil sharpeners? What kind of pencil sharpeners? What gearhead said is dead true. You just have to keep your eyes open. Its amazing
the amount of stuff I spot when I'm not looking for anything in particular.
[Edited on 4-9-2010 by cnidocyte]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
If price is no object
http://www.admackay.com
daily market values
http://www.metalprices.com
.
|
|
|
Random
International Hazard
    
Posts: 1018
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by cnidocyte  | Zinc - The casing of the cells in zinc chloride batteries
Manganese dioxide - Found inside the zinc casing of the cells in zinc chloride batteries
Zinc chloride - Mixed in with the manganese dioxide
Platinum - Some jewellery making shops sell platinum wire
Palladium - ^^
Silver - ^^
Gold - ^^
^^ = same as above
Magnesium from pencil sharpeners? What kind of pencil sharpeners? What gearhead said is dead true. You just have to keep your eyes open. Its amazing
the amount of stuff I spot when I'm not looking for anything in particular.
[Edited on 4-9-2010 by cnidocyte] |
Thanks for the information, magnesium can be obtained from KUM (made in Germany) pencil sharpeners . They say magnesium on them sometimes and it
really is (I dissolved the metal in citric acid.)
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Pottery supply stores, seriously, they have tons of useful chemicals in bulk for bulk prices at a nice purity. Find one nearest you 
|
|
|
Doktor Klawonn
Harmless

Posts: 29
Registered: 20-11-2010
Location: Europe
Member Is Offline
Mood: No Mood
|
|
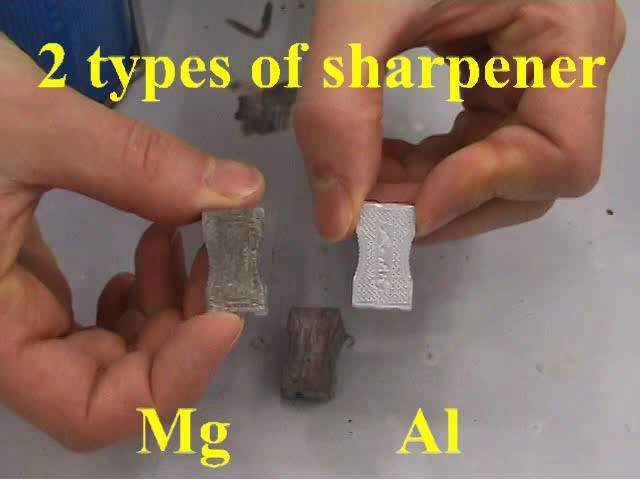
Those little ones, made fully from metal. There are two types of them, both look very similar. One type is of aluminum the other of magnesium. See the
attached picture: The left and the middle one are from magnesium.
Those Mg sharpeners can be ignited by a decent burner, please take a look at this video.
Dr. K.
|
|
|
cyanureeves
National Hazard
   
Posts: 737
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
salts
your garden center will have many kinds of salts. copper sulfates.iron sulfate, i made potassium chlorate from muriate of potash. urea is there
also.so is kno3. ammonium nitrate from walgreen(instant ice pack)to mix with sulfuric acid to make more metal salts. magnesium sulfate at grocery if
youre nickel plating.nicad batteries, lithium batteries for lithium..sodium metabisulfite from wine makers supply.the first ammonia i made was heated
with one of those light bulbs.canadian quaters made before 2000 are nickel. titanium from light weight camping cooking pans.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Scrap yards will have many metals on hand since it's their job to isolate them from sources. I've purchased relatively pure nickel, lead, magnesium,
aluminum, tungsten, silver, and copper from them at slightly above market price.
|
|
|