Simoski
Hazard to Self
 
Posts: 82
Registered: 24-12-2017
Location: Johannesburg South Africa
Member Is Offline
Mood: No Mood
|
|
Electrochemical cell math
Hello
I'd love to understand the math of electrochemistry.
Maybe build us an online calculator or something like that.
Would someone please teach it to me or give me good resources.
I run 3 electrochemical cells, 2 potassium chlorate & 1 sodium chlorate... What measurements can I do on them with a multimeter and what math can
I do on those measurements to get the current efficiency ? What Faraday laws do I need to understand to predict production in grams per hour?
8 )
Thank you in advance
Simoski
PS maybe asking what the relationship between the Faraday laws and electrochemical chlorate production is, would have been a better way of asking it.
[Edited on 24-2-2019 by Simoski]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
The net electrochemical equations for chlorate production:
At the anode:
3 H2O + Cl- + -> ClO3- + 6 +H + 6 e-
At the cathode:
6H+ + 6 e- -> 3 H2
At maximum efficiency, it takes six moles of electrons moving through the cell to produce one mole of chlorate. Faraday's constant is 96485 C/mol e-
so this is equivalent to 578910 C per mole chlorate. Note that 1 C = 1 amp * 1 s
Of course, a real cell won't operate at maximum efficiency, so it will take somewhat more than this.
|
|
|
Simoski
Hazard to Self
 
Posts: 82
Registered: 24-12-2017
Location: Johannesburg South Africa
Member Is Offline
Mood: No Mood
|
|
Hi Metacelsus
Thanks for your reply.
How do I measure the voltage drop across the cell?
I mean do I measure with the power on or off?
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Measure the voltage with the power on. But you should also measure the current since that will tell you the rate of chlorate production.
|
|
|
Simoski
Hazard to Self
 
Posts: 82
Registered: 24-12-2017
Location: Johannesburg South Africa
Member Is Offline
Mood: No Mood
|
|
So with the supply on, I measure the voltage at the supply terminals.. then the voltage drop across the cell array (2 in series) is the supply voltage
minus the measured voltage?
If I decouple the cell array from the supply and measure the resistance, can I then use ohms law to calculate the current? Or must I add a resistor of
a know value in series and measure the voltage drop while the array is running?
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
The best way to do it is with a small resistor (say, 0.1 ohm) in series. Measuring the cell resistance when it's off will be misleading since the
current-voltage response will not be linear. Some multimeters also allow current measurements directly.
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Voltage supplied is largely dependent on your power supply. The resistance and hence current of a cell can vary during operation. For a feeble
supply this can cause the voltage to fluctuate also but if you have a decent source you should be ok.
The alternative is to have a current-regulated power supply -- which is merely a way of adjusting the voltage to maintain a constant current.
Current is the main parameter for an electrochemical cell. It determines the rate that you make your product.
Yield = tI/(Fn)
yield is in moles
F = 96485
t is time in seconds
I is current in amps. (If you don't have a current regulated supply, you take regular measurements and figure out an average.)
n is the number of moles of electrons needed for each mole of product.
There are of course improvements you can do to make your actual yield close to the theoretical.
1. Keep current density low to preserve electrodes. This means large electrodes or long times.
2. Operate with as low as possible overpotential. This increases inefficiencies.
3. Reliable power supply. I love my current regulation.
4. Specific conditions that relate to your particular reaction. In the case of chlorates, from memory, 70°C and a touch of dichromate.
|
|
|
Simoski
Hazard to Self
 
Posts: 82
Registered: 24-12-2017
Location: Johannesburg South Africa
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  |
Yield = tI/(Fn)
yield is in moles
F = 96485
t is time in seconds
I is current in amps. (If you don't have a current regulated supply, you take regular measurements and figure out an average.)
n is the number of moles of electrons needed for each mole of product.
|
Thanks J : )
This looks to be the basis of a calculator... right?
[Edited on 2-3-2019 by Simoski]
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
https://geocitieschloratesite.000webhostapp.com/chlorate/run...
top is https://geocitieschloratesite.000webhostapp.com/chlorate/chl...
Understand what is a mole and a mole of electricite and you done. Done obsess over voltage
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have written webpages about electrolysis and a powersupply:
http://woelen.homescience.net/science/chem/exps/electrolysis...
http://woelen.homescience.net/science/chem/misc/psu.html
These links may make things easier for you to understand.
The current through a cell is what matters. By keeping resistance low you can keep power efficiency high. Current efficiency is another thing than
power efficiency. Current efficiency is the amount of current, which leads to the desired chemicals, relative to the total current. Current efficiency
can be 100% while power efficiency is low (if all current leads to formation of the desired chemicals, but the voltage across the cells is high). A
less than 100% current efficiency is due to side-reactions which lead to other products than the desired ones.
The following link covers current efficiency in a chlorate cell:
http://woelen.homescience.net/science/chem/exps/miniature_ch...
|
|
|
Simoski
Hazard to Self
 
Posts: 82
Registered: 24-12-2017
Location: Johannesburg South Africa
Member Is Offline
Mood: No Mood
|
|
Thank you Woelen, Gentlemen!
Woelen I particularly enjoy your model of the chlorate cell as the following:
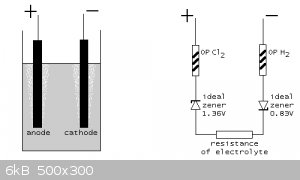
[Edited on 30-3-2019 by Simoski]
|
|
|
Simoski
Hazard to Self
 
Posts: 82
Registered: 24-12-2017
Location: Johannesburg South Africa
Member Is Offline
Mood: No Mood
|
|
Guys when I disconnect my chlorate cell from the power supply and measure the galvanic voltage ( around 3.5 volts ) is this galvanic voltage the EMF
of the cell?
In the beginning of a chlorate cell run I'd imagine that this galvanic voltage is near zero. Just chloride in solution, only later when the cell has
been running for a while and clo- , clo2- and clo3- ions have been created, will a galvanic voltage be present.
Any comments or thoughts?
|
|
|