Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
pressure problem
What is the absolute pressure in a closed vessel at equilibrium if liquid water is present, the temperature is 178.3C, and the vapor is 50mole%
water/50mole% air?
I prefer the answer in psia, but kPa will be OK.
I have calculated the answer but it is contradicting my observation, so I'd like your conclusion.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
It will be the vapor-pressure of the water at that temperature ; the air plays no role: It gets compressed to the vapor-pressure of the liquid ...
which depends on the temperature, but not the mole-%age of any present air ...
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
~140 psi, and I agree with chief - if there is equilibrium then the temperature of the water should be all you need to know...
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
I'll assume two physical equations: the ideal gas law PV = nRT and Dalton's law of partial pressures P = Σp<sub>i</sub>. For the
water n/V = P/RT, and you have P for water from its vapor pressure and we're given the temperature. Because we have a single chamber, V is identical
for air. Clearly the temperature is the same. You said explicitly that the molar fractions are equal. Therefore the partial pressure of the air is the
same as that of the water. The total pressure is twice the vapor pressure of water at that temperature.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
My solution was the same as watson's. This means that when air is mixed with the water vapor in a sealed container the indicated pressure should be
higher than what one would get from a steam table for saturated steam.
This is just the opposite of what I'm seeing on my autoclave as I do shakedown runs with water. Now that we've eliminated air as a culprit I'm back
to wondering what causes this discrepancy. It is possible that lack of equilibrium is involved.
Below is a representation of my data:
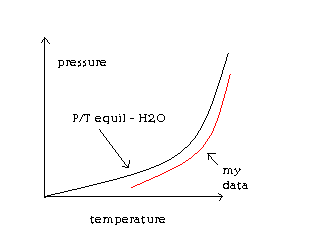
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | Now that we've eliminated air as a culprit I'm back to wondering what causes this discrepancy. It is possible that lack of equilibrium is involved.
|
It look like garden-variety deviations from ideal gas law, which is large for steam, particularly at
autoclave temperatures and pressures. I'd recommend getting a copy of the known steam data.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
That's what I used to construct the P/T equilibrium curve.
I checked the calibration of the thermometer and it's good.
I haven't checked the calibration of the PI yet but it is a brand new WIKA (0-1000 psi) with ss316 wetted parts.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by watson.fawkes  | | I'll assume two physical equations: the ideal gas law PV = nRT and Dalton's law of partial pressures P = Σp<sub>i</sub>. For the
water n/V = P/RT, and you have P for water from its vapor pressure and we're given the temperature. Because we have a single chamber, V is identical
for air. Clearly the temperature is the same. You said explicitly that the molar fractions are equal. Therefore the partial pressure of the air is the
same as that of the water. The total pressure is twice the vapor pressure of water at that temperature. |
This assumption works for gas-only systems ... ; as long as water can condense ... it will so ...
==> and enough water will be a non-gaseous liquid to prevent the pressure going above the vapor-pressure at the given temperature ...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
My understanding is that equilibrium is established when the partial pressure of the water vapor is equal to the vapor pressure of
the liquid water. Since there are equal moles of air the partial pressure of the air equals that of the water vapor. Therefore, the
total pressure = 2 x vapor pressure of water at the given temperature.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Pressure will equilibrate much faster than
temperature. You're seeing results as if your temperature were lower. The average temperature may indeed be lower if you're cooling off significantly
somewhere, like the fill pipes. You can think of your thermometer as a one-point sample of the temperature field. If you're actually in equilibrium
that sample will suffice. Away from equilibrium, it may not.
For a test, put the whole thing in an insulating box that itself has constant temperature.
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | | My understanding is that equilibrium is established when the partial pressure of the water vapor is equal to the vapor pressure of
the liquid water. Since there are equal moles of air the partial pressure of the air equals that of the water vapor. Therefore, the
total pressure = 2 x vapor pressure of water at the given temperature. |
Pressure is pressure, no matter by which molekules it is exerted ... ; so at the interface between the liquid and the gases the water will not give
away more gas-molecules then it could at the given pressure ... no matter by which gases (air) it is exerted ...
As an example may serve the boiling of eggs in the himalaya-mountains ... where the water boils at below 80 [Cels] ... and at sea-level, where the
water boils at 100 [cels] ...
==> The water-surface reacts to the outer pressure ... ; it doesn't matter that it stems from air ... ; it would be the same boiling-temperatures
for any other air-nitrogen-mixture ...
=================
Another example: If you were right ... then you could fill up a bottle with a mixture of a lot of liquids ... ; all the partial pressures of those
would add up ... giving potentially a _lot_ of pressure ...
==> But that is not observed ...
An experiment might be done: Gasoline has a quite high partial pressure: When opening a bottle with some of it ... it gives a little blowout ...
==> One might measure this pressure under different amount of air present in the bottle (U-tube-manometer) ... : I bet the pressure depends only on
the temperature, not on the amount of air inside ... : The pressure will be the same whether the bottle is filled to 20 % or 80 % ...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
That is true. "Partial pressure" is a useful mathematical construct, very widely used.
Quote: Originally posted by chief  |
Another example: If you were right ... then you could fill up a bottle with a mixture of a lot of liquids ... ; all the partial pressures of those
would add up ... giving potentially a _lot_ of pressure ...
==> But that is not observed ...
|
But that is observed. These are the methods used for calculating the vapor composition in equilibrium with a solution of two or more components, eg,
benzene and toluene. See Raoult's Law and Dalton's Law of Partial Pressures.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
So, what are your results, when you remove all of the air, prior to heating your autoclave?
Where is the thermometer sensor located? In the liquid phase, or the gas?
You do of course, have your autoclave well insulated?
----------------------------------------------------------------------------------------------------------
More importantly, how did you build your autoclave? Can we do it too?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by zed  | So, what are your results, when you remove all of the air, prior to heating your autoclave?
Where is the thermometer sensor located? In the liquid phase, or the gas?
You do of course, have your autoclave well insulated?
More importantly, how did you build your autoclave? Can we do it too?
|
The autoclave is per my design and therefore as yet unproven. I'm proving its operability by conducting a series of increasingly more challenging
tests. This started with a 1500 psi hydrostatic test, previously described.
I did boil my water before loading the autoclave to eliminate dissolved gas. Once the autoclave is sealed there is no simple way to get the
headspace air out. This would be 72mL out of a total capacity of 372mL. Boiling the water in the autoclave as the final plug is installed would have
been the best way, I guess. I didn't know I had this anomaly until I started the water heating tests.
The Hg-glass thermometer fits snugly into a thermowell. The thermowell is submerged 3" at 300mL of water. A little silicone oil was poured into the
well to serve as heat transfer fluid.
The upper part of the autoclave is extremely well insulated and is fitted with an auxiliary electrical heater. The bottom part is in a temperature
controlled hot oil bath.
See the text in the library on autoclaves. I built mine to the most basic of these designs. In theory anyone can build one of these, especially once
you have a proven design. Please note the reminder at the bottom of my posts. This mostly reminds me of my significant toll in time and money. Time
and money will decrease somewhat once the development has been completed.
[Edited on 2-10-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
I don't know if you find the answer, but I am sure that is not 140 psi...
In my book of thermodynamique (A pragmatic approche) I have some tables that give the pressure of water compare with the temperature.
The important thing is that you are in the saturate liquide phase.
So this is true for 95%/5% or 5%/95% of liquid/vapor.
Two important point:
175 C ---> 892,60kPa
180 C ---> 1002,80 kPa
for PSI;
892,60 / 14,7 = 65,67 PSI
This is true at equilibrium. (pressure is fast but temperature can be long if there is no mixing)
With some approximation ; (this is not linear...)
178,3 C --> 965,332 kPa
P.S.
PV=nRT can be use for water vapor at very high temperature and at lot pressure.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yep, right out of the steam tables for saturated steam, where no air is present.
No, divide by (101.325/14.7) = 6.89. Therefore the kPa you gave above convert to 129.5 and 145.5 psi, respectively.
That was just a hypothetical problem to verify which direction the pressure should go from a saturated condition when air is present. My data went
the other direction and I still don't have an explanation.
Now here is a real problem with air and water:
What is the pressure in the vapor space when the vapor space is composed of 97.54mole% air and 2.46mole% water, and is in equilibrium with liquid
water at 21.1C?
[Edited on 3-10-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
You are right....My bad...The good point is that i did good in the last week exam . .
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | | What is the pressure in the vapor space when the vapor space is composed of 97.54mole% air and 2.46mole% water, and is in equilibrium with liquid
water at 21.1C? |
Assuming that air is insoluble in water (only approximately true), you need to specify one
of the following: the volume of the vapor space, or the mole quantity of either water vapor or air.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Are you sure about that watson? This is right from a psychrometric chart.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
No you're right. I was solving
some other problem. Even at this short remove, I can't tell what problem that was. I think I must keep drifting off to your autoclave, where you can't
just go measuring mole percentages in the headspace.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This last problem can be solved as follows, using the same principles as those used for the hypothetical problem above having 50mole% air:
vapor pressure of water at 21.1C (70F) = 18.8mmHg
Since the water vapor/air mix is in equilibrium with liquid water, the partial pressure of the water vapor is also 18.8mmHg.
The the total pressure of the gas/vapor mix must be = (100/2.46)18.8 mmHg = 764mmHg, or~1atm. This agrees with a data point on the 100% relative
humidity curve found on the psychrometric chart.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Here's some results from a another heatup test of the autoclave, this time using methanol. Also, the gasket material was changed from Aramid
fiber/nitrile rubber to graphite.
This pressure vs temperature data, shown below, looks much better. The continuous curve is plotted from CRC handbook data. The red data points were
taken on heatup, and the black squares were taken during cool down.
I think that the discrepancies on heatup are due to temperature inequality of the head flange/thermowell and the main body. The main body is heated
with an oil bath and the upper flanges, which hold the thermowell, are heated with an auxiliary heater. I believe that the good conductivity of the
graphite (~130W/m-K) helped the parts reach temperature equality (The thermal conductivity for steel is ~30W/m-K.)
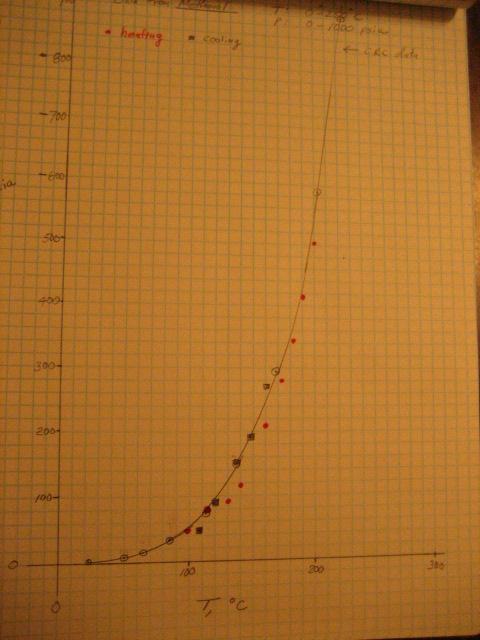
[Edited on 6-10-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|