| Pages:
1
..
4
5
6
7
8
..
40 |
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Awesome, keep up the pretty pictures!
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Funny, that. Now your FeCl3.6H2O looks much darker than mine:
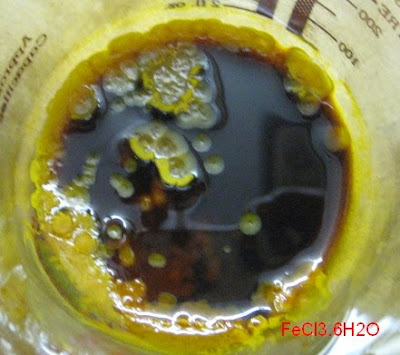
Yours looks a bit hydrolysed…
[Edited on 27-5-2011 by blogfast25]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by LanthanumK  | Here are some pictures of cobalt compounds I synthesized for the purpose of photography. They are some of the more colorful compounds I photographed.
|
What compound is the one on the left? Really nice granular crystals, and the deep red is beautiful!
|
|
|
Megamarko94
Hazard to Self
 
Posts: 68
Registered: 31-12-2010
Member Is Offline
Mood: No Mood
|
|
my fecl3 was a bit to long in a desicator...
|
|
|
Megamarko94
Hazard to Self
 
Posts: 68
Registered: 31-12-2010
Member Is Offline
Mood: No Mood
|
|
i think its cobalt (II) chloride hexahydrate...
http://en.wikipedia.org/wiki/Cobalt%28II%29_chloride
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Not a very sharp shot: phthalic acid needle-like crystals obtained by re-crystallisation of crude phthalic acid, now sitting in an ice bath. The
phthalic acid was obtained by alkali hydrolysis of di (2-ethylhexyl) phthalate (commonly: DOP) and subsequent neutralisation of dipotassium phthalate.
The DOP was extracted from vinyl (pPVC) gloves). These took several hours to materialise:

Diameter of outer circle about 5 cm.
[Edited on 27-5-2011 by blogfast25]
|
|
|
LanthanumK
Hazard to Others
  
Posts: 298
Registered: 20-5-2011
Location: New Jersey
Member Is Offline
Mood: No Mood
|
|
Here are more pictures. Many of my other pictures cannot be uploaded though because of the 800 pixel limit.
  
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
And these are?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
If you place the cursor over the image then it tells you...
|
|
|
LanthanumK
Hazard to Others
  
Posts: 298
Registered: 20-5-2011
Location: New Jersey
Member Is Offline
Mood: No Mood
|
|
Sorry if I wasn't clear. 
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Hi Here are a few pretty coloured materials
1. Mercuric iodide
2. Stannic iodide in cyclohexane
3. Cobalt Thiocyanate in acetone
4. Not pretty, but some ampouled sodium I made years ago.
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
Stannic iodide (SnI4)? Or stannous iodide (SnI2)? Because stannic iodide is orange, and the stannous one is usually red.
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 857
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
I'm guessing it's tin(II) iodide, considering that tin(IV) iodide is readily soluble in apolar solvents such as cyclohexane.
Edit: I see that nezza said it's stannic in his post. Now I'm not sure any more.
[Edited on 28-5-2011 by Lambda-Eyde]
This just in: 95,5 % of the world population lives outside the USA
You should really listen to ABBAPlease drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
condennnsa
Hazard to Others
  
Posts: 217
Registered: 20-4-2010
Location: Romania
Member Is Offline
Mood: No Mood
|
|
I don't know where to post this and don't want to open a new thread, also the crystals are quite pretty.
I added 200ml 35-40 % H2SO4 , 200 ml water and 100g KNO3 to a 2L beaker and 135 g copper wire.
After the vigorous reaction stopped, which gave off large amounts of NO2 gas, and the solution cooled these crystals formed
And I'll be damned, these look nothing like copper sulfate crystals! even though they're blue, as you can see, they're sky blue, not the well know
copper sulfate blue. And I have seen and grown copper sulfate crystals many times, these are a totally different color! Could it be a double salt?

[Edited on 2-6-2011 by condennnsa]

[Edited on 2-6-2011 by condennnsa]

|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Its normal for the different salts to have different colours. Copper (II) acetate is also different, being a darker blue/green.
|
|
|
Squall181
Harmless

Posts: 46
Registered: 21-2-2011
Member Is Offline
Mood: No Mood
|
|
Copper Nitrate Crystals in a Beaker
Iron(II) Oxalate, KMnO4, Copper Carbonate
Copper Crystals
  
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by condennnsa  | I don't know where to post this and don't want to open a new thread, also the crystals are quite pretty.
I added 200ml 35-40 % H2SO4 , 200 ml water and 100g KNO3 to a 2L beaker and 135 g copper wire.
After the vigorous reaction stopped, which gave off large amounts of NO2 gas, and the solution cooled these crystals formed
And I'll be damned, these look nothing like copper sulfate crystals! even though they're blue, as you can see, they're sky blue, not the well know
copper sulfate blue. And I have seen and grown copper sulfate crystals many times, these are a totally different color! Could it be a double salt?
|
Did you check whether it was effectively copper sulphate? Test with CaCl2 for instance.
Try and recrystallise a small amount?
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by condennnsa  | I don't know where to post this and don't want to open a new thread, also the crystals are quite pretty.
I added 200ml 35-40 % H2SO4 , 200 ml water and 100g KNO3 to a 2L beaker and 135 g copper wire.
After the vigorous reaction stopped, which gave off large amounts of NO2 gas, and the solution cooled these crystals formed
And I'll be damned, these look nothing like copper sulfate crystals! even though they're blue, as you can see, they're sky blue, not the well know
copper sulfate blue. And I have seen and grown copper sulfate crystals many times, these are a totally different color! Could it be a double salt
|
Perhaps it's copper nitrate? That's how you make nitric acid after all, by combining sulfuric acid and a nitrate salt. Since you didn't have
concentrated sulfuric there probably wasn't a lot of this produced, but the color and shape of your crystals looks a bit like copper nitrate to me.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
No it's likely to be K2SO4 or KHSO4, wich looks blue due to some cocrystallised CuSO4.
[Edited on 2-6-2011 by Jor]
|
|
|
condennnsa
Hazard to Others
  
Posts: 217
Registered: 20-4-2010
Location: Romania
Member Is Offline
Mood: No Mood
|
|
Well, I've since done more reading around the internet, and I think that this is indeed a double sulfate of copper and potassium. Some stuff I found:
http://dalspace.library.dal.ca/bitstream/handle/10222/12491/...
This is apparently a paper from 1898, they give a thorough preparation of this salt, as well as an analysis, which they say is CuK2(SO4)2 * 6H2O.
http://books.google.com/books?id=HKgMAAAAYAAJ&pg=PA529&a...
Britannica also mentions this double salt :
" Cupric sulphate crystallises with 5 molecules of water, but it forms a double sulphate with potassium sulphate, CuK2(SO4)2 . 6H2O, isomorphous with
the corresponding zinc and magnesium salts.
I find finds like this very exciting , even though i'm just an amateur who likes to read this and that about chemistry and I don't really understand
many of the more advanced underlying phenomena, the sight of these beautiful crystals bring me lots of joy. Copper has such a rich chemistry.
I initially intended to use this procedure to precipitate blue undecomposed Cu(OH)2, but now I think I'll keep the double salt.
[Edited on 2-6-2011 by condennnsa]
Update - I added 100ml more 35-40% H2SO4 and 100g KNO3 and 300ml water to dissolve more of the copper, I heated the solution to get the reaction
going. After a long time it , there is not much left of the copper wire, and upon cooling down the same crystals formed this time more numerous and
smaller:


-Being the idiot that I am, I forgot to change the white balance on my camera, it was set for daylight, that's why the crystals appear more greenish.
In fact they're the same color as in the previous pictures.
[Edited on 2-6-2011 by condennnsa]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Nice bit of Netresearch there, Condenssa. It looks like the double salt CuK<sub>2</sub>(SO<sub>4</sub> <sub>2</sub>.6H<sub>2</sub>O may well be the compound you
stumbled upon. It would be nice to have analytical certainty though. Determining the amount of crystal water would be one element of analysis that
could confirm (or infirm) the hypothesis that your stuff is indeed CuK<sub>2</sub>(SO<sub>4</sub> <sub>2</sub>.6H<sub>2</sub>O may well be the compound you
stumbled upon. It would be nice to have analytical certainty though. Determining the amount of crystal water would be one element of analysis that
could confirm (or infirm) the hypothesis that your stuff is indeed CuK<sub>2</sub>(SO<sub>4</sub> <sub>2</sub>.6H<sub>2</sub>O. For this, weight accurately some
of the dry hydrate crystals, then drive off the water by heating and weigh again. From the weight loss we could then determine if the hydrate water at
least corresponds to the alleged formula. <sub>2</sub>.6H<sub>2</sub>O. For this, weight accurately some
of the dry hydrate crystals, then drive off the water by heating and weigh again. From the weight loss we could then determine if the hydrate water at
least corresponds to the alleged formula.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
  
I was reducing a solution of (NH4)2PdCl6 (about 400mg Pd) in 25mL of 10-15% ammonia with hydrazine sulfate at 60-70C. A beautiful palladium mirror
formed, but I had no camera at hand. Shame, would have been a nice picture!
Quite a nice day of experimenting, I also harvested about 2 grams of (NH4)2PtCl6  , made 5 grams of pure white needles of p-bromoacetanilide and am currently making bromobenzene by rxn of KBrO3 in dil. H2SO4 with benzene.
, made 5 grams of pure white needles of p-bromoacetanilide and am currently making bromobenzene by rxn of KBrO3 in dil. H2SO4 with benzene.
[Edited on 3-6-2011 by Jor]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
These copper crystals are awesome! How did you do that? Did you make them by means of electrolysis? Please provide us with details about the process.
|
|
|
Squall181
Harmless

Posts: 46
Registered: 21-2-2011
Member Is Offline
Mood: No Mood
|
|
@ Woelen Yes those where made by electrolysis of copper sulfate solution with a copper anode and copper cathode.
Those crystals where grown in the course of a week with a pretty dilute solution of copper sulfate.
A 12 volt adapter that could supply 1 amp of current was used; I did not have a multimeter on hand to take exact measurements.
The rig is pretty simple to build: a 20 oz soda bottle is taken and the top cut off, then the top is inverted back into the bottom piece of the bottle
and taped with the cathode down in the bottom of the bottle. Next a filter is made using paper towel or something similar and is placed into the
inverted cone. A stiff piece of wire is used at the top as a connection from which a copper anode is hung. The power is switched on and you have to be
really patient, because it is a slow process, but I think if you use a more concentrated solution it should be faster.
Here is a link to where I got the idea: http://youtu.be/kq1W-QdMsWQ
Skip to the middle to see the how the cell is constructed.
|
|
|
Rogeryermaw
National Hazard
   
Posts: 656
Registered: 18-8-2010
Member Is Offline
Mood: No Mood
|
|

this is NH4NO3 recrystallized from cold packs.
http://www.sciencemadness.org/talk/viewthread.php?tid=16374&...
|
|
|
| Pages:
1
..
4
5
6
7
8
..
40 |