| Pages:
1
..
21
22
23
24
25
..
40 |
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
This is a photo of about two grams of manganese(III) acetylacetonate prepared by manganese(II) sulfate and acetylacetone. The oxidation to Mn(III) was
carried out using potassium permanganate

|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Very nice, Doug!
What be you distilling?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
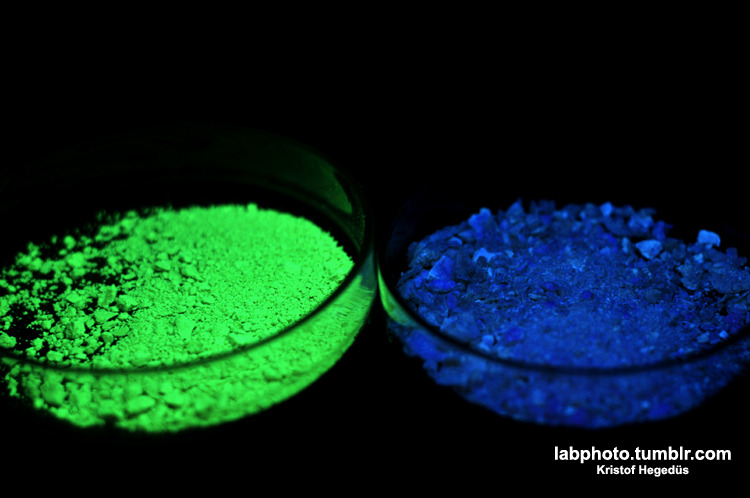
Left side: my starting material, a highly substitued pyrrole (also prepared by me), right side: the compound what I have made from it. Both are under
UV.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Bloody hell, that's pretty amazing, kristof.
What are the details of the compounds you used?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
Just running a finishing pass on about .750L of fresh HNO3 to bring it to 68% azeotropic. The sulfuric acid was used in the initial run in conjunction
with a whole bunch of CAN fertilizer.
Victor Grignard is a methylated spirit.
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
http://www.flickr.com/photos/89521313@N06
This is a picture of NO2 dissolved in chloroform. At room temperature, it is this bright yellow colour, which turns to a deep orange at higher
temperatures.
My photography skills are crap, but I still think this is pretty good.
Edit: Directly posting the image somehow doesn't work, so I changed that to a link.
[Edited on 1-11-2012 by weiming1998]
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Here are chlorine bubbles through olive oil. This was my attempt to isolate chlorine gas by electrolysis. I was hoping that olive oil would be inert
enough not to react too much with chlorine, but it ended up reacting anyway. If you look closely at the top of the column, you'll see white streaks
where the oil reacted with the chlorine. At the beginning, the oil was perfectly clear.
[edit] the additional picture is not pretty, but I included it to show the extent of the chlorination.
 
[Edited on 11-1-2012 by White Yeti]
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
That is really cool yeti! I might try that just for fun! 
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Wtf? Oleic chloride?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Time for Tin! (again)
I made more tin crystals from an acidic solution of tin chloride- normally you add granular zinc to the solution to start the reaction and create the
"floating tin sponge"
This time I wanted to try with a zinc rod which was too heavy for the bubbles to lift, It made a beautiful tin tree type thing.
The solution is ~0.5 M in SnCl2 and 2 M in HCl
  
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Addition of Cl2 across the double bonds in the triglycerides.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Oh yeah, now it makes sense. By the way, what a pretty tin picture, that last one he made.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
I wish I had something better than an iPhone5 to take pictures, every time I try to show you guys something it turns out shittier than my eyes see it!
Thanks though 
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
It's not bad Mailin'. [Brace yourself for constructive criticism ] one parameter
you could improve is the lighting. ] one parameter
you could improve is the lighting.
The more light available for the picture, the better it tends to turn out. Just make sure you don't go overboard either.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by White Yeti  | It's not bad Mailin'. [Brace yourself for constructive criticism ] one parameter
you could improve is the lighting. ] one parameter
you could improve is the lighting.
The more light available for the picture, the better it tends to turn out. Just make sure you don't go overboard either. |
Not bad- could be better  I actually did the reaction on a windowsill thinking
that the bright daylight would show off the crystals more, but it didn't, so I had to use flash which made it.... Okay. I actually did the reaction on a windowsill thinking
that the bright daylight would show off the crystals more, but it didn't, so I had to use flash which made it.... Okay.
iPhone are not the masters of photography I have to admit, next time I will try a light behind me or something. I plan to grow some electrolytic tin
crystals, but I will figure out my lighting before I do it, for sciencemadnesses' sake 
Thanks for the criticism though, I love taking pictures of what I do, I just need to perfect doing it with what I have!
[Edited on 3-11-2012 by Mailinmypocket]
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Taking pictures is a rather subjective process, everyone has their own style. I like to get light to come in through the side. But in some cases, I
don't have the choice, and I need to use flash. This might have been the situation you ran into.
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Carborundum (silicon carbide), and it's fairly old container. Very hard and extremely shiny little bits- glass can easily be scratched with a granule of this
stuff...

|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Here is a video I made that demonstrates precipitating gold from chloroauric acid solution using boiling oxalic acid as the precipitant;
http://m.youtube.com/watch?v=o165tgxFMYM
Kadriver
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Fine Silver 5 Troy Ounce Bars
Bathing in dilute sulfuric acid to remove all traces of flux from the melt.
Each of these bars weigh between 5.020 and 5.040 troy ounces.

|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Stamped Bar
After having the fine silver crystals (grown in my electrolytic silver cells) assayed by NTR Metals, I hand stamp the bars.
These sell on Ebay for 5 to 10 percent over spot price.
After Ebay fees, I net right at spot for each of these bars.
kadriver

|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Platinum Leach from Catalytic Converters
Here is a shot of some platinum and palladium in solution from leaching automotive catalytic converters in hydrochloric acid and clorox.
The solution is course filtered via gravity first using several coffee filters to remove large particulate from the solution.
Then it is filtered a second time using vacuum assist through a whatman number 5 fine filter paper.
kadriver - Edited once to correct a spelling error.

[Edited on 13-11-2012 by kadriver]
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Platinum Group Metals Mixed Black Powder
This is the platinum group metals that have been cemented (precipitated) using pure zinc metal shavings.
The zinc shavings were added to the platinum/palladium solution and the metals "cement" into the zinc trading places as the zinc goes into solution,
and the platinum groups metals precipitate out of the solution.
See "electromotive series" or "replacement series" for an explaination of how this works.
These mixed (platinum, palladium, and probably rhodium) black powders are pure metal. But they will need to be refined (seperated) from each other
using some complex chemistry (of which I know very little).
kadriver

|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
Pure Gold Bar 1.7 Troy Ounce 99.5% Pure
Here is a pure gold bar that I produced using chemistry and metalurgy to remove impurities from the gold (10k and 14k scrap jewelry).
The scrap gold was alloyed with scrap 925 silver then treated with dilute nitric acid to dissolve most of the base metals - nitric acid will not
dissolve gold, but it will dissolve out the base metals.
The resulting gold was then dissolved using a mixture (4 to 1) of 31.45% hydrochloric acid (from Lowes) and concentrated (68%) technical grade nitric
acid.
After removing any excess nitric acid and filtering, the pure gold powder was precipitated out of solution using a saturated, and filtered solution of
sodium metabisulfite.
There is a tiny bit of contamination seen as tiny bumps and irregularities on the surface of the bar to the left.
To get this bar up to spec, I would have redissolved the gold powder in hydrochloric acid and clorox (from the Dollar Store), then precipitated again
using boiling oxalic acid (99.9% ACS) to produce a bar in the range of 99.99% purity.
This bar was sold to a big refinery, so I did not waste the time and chemicals to refine it all the way to industry standards of 99.95% purity.
Thanks for looking.
kadriver
 
|
|
|
kadriver
Hazard to Others
  
Posts: 196
Registered: 7-11-2012
Location: United States
Member Is Offline
Mood: Thankful
|
|
I posted the above using an ipad and it does not work with a PC.
Here is the link that will work with a PC;
http://www.youtube.com/watch?v=o165tgxFMYM
Thanks for looking.
kadriver
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
I2 that has been melted under air and then solidified (I was recrystallizing it and had to stop)
[Edited on 14-11-2012 by Pyro]

all above information is intellectual property of Pyro.  |
|
|
| Pages:
1
..
21
22
23
24
25
..
40 |