| Pages:
1
2
3
4
5 |
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I had solution of hypomanganate in fridge stable for at least 20 minutes. I wasn't really examine stability of solution, I just dilute it and pour in
to the drain. But now it's interested me, I will let you know when I test it.
In my experience - mixed solution of manganate and permanganate have dark blue colour (see starting chemical chameleon or try put excess of
permanganate to alkaline solution of thiosulfate). But this is precipitate - this may be different.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I made K2Cr3O10 and ampouled this.
I made this chemical by dissolving K2Cr2O7 and some CrO3 in boiling hot highly concentrated HNO3 (70% or so, maybe even a little more). The nitric
acid I made by distillation from H2SO4 and KNO3 and a little water to make really concentrated acid. In such concentrated acid an amazingly large
amount of K2Cr2O7 can be dissolved and when that is done, then it also is possible to dissolve some CrO3 in it as well. In order to do so, the liquid
must be boiling hot, otherwise you don't get all of it dissolved.
After that, I put the flask with the acid and dissolved chromium compounds in a hot water bath, which I slowly let cool down. The next day I had a big
crop of beautiful red K2Cr3O10. I put this on a glass fritte and used some paper tissue on the other side of the fritte to absorb the acid and any
CrO3 which still is dissolved in it. This was quite scary, the acid with CrO3 is a very harsh oxidizer and after ten seconds or so, the paper tissue
with acid soaked in it starts to fume and hiss. So, I had a bucket with water nearby and dumped the paper tissue with absorbed acid in that
immediately. I repeated this procedure until no more acid could be absorbed in paper tissue. At this point, the solid already was quite dry.
Next, I put the solid in a petri dish and put it in an oven at 100 C for 20 minutes or so. I finally put the petri dish in a plastic jar, in which I
also put a small dish, full of NaOH. This absorbs acid vapor and after several days I had a perfectly dry product, which could be ampouled nicely.
Below follow pictures of the ampouled product on a white background and on a black background:


I also made pictures where the compound is kept near the container of my K2Cr2O7. These pictures nicely show the difference between K2Cr2O7 and
K3Cr3O10.


Once the K3Cr3O10 is completely dry and free of acid, it is not hygroscopic anymore, and it can be kept around very well. I will use this sample in my
element collection as an addon to the chromium sample (together with Cr2O3, CsCr-alum, K2CrO4, K2Cr2O7).
[Edited on 18-5-19 by woelen]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Very nice! I'm also curious what it would look like next to CrO3. Is it an intermediate between the oxide's dark red and dichromate orange?
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
It indeed is an intermediate color. CrO3 is darker.
I can make a picture of K2Cr3O10 besides some CrO3. Expect that picture one of these days . . .
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Interesting compound with beautiful colour. Sadly I saw this compound only on pictures  . .
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
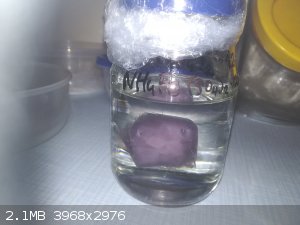
Hi.
Here is photo of one of my crystals of ammonium-ferric sulfate. Crystal weight is 37,7g. On photos has beautiful pale violet colour but currently is
transparent on edges (but middle is still pale violet) and surface is little bit rusty but it doesn't become rusty even more. I store it in paraffin
oil to prevent water losses (on the air it lose water and becomes rusty).
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|

Figure 1: I started stockpiling orange peels in my freezer, telling myself that I'd steam distill them some day. By the time the freezer was full
& I got around to the project, I had 2.63 kg hoarded.

Figure 2: When I was done, I had 41.80 g of orange oil collected, for a dry mass percent of ~1.6% oil. I measured its density as 0.837 g/mL.
Also, my house smelled like Froot Loops the whole time 
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
@mayko
Nice! That's the largest batch of limonene extraction I've ever seen!
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
That's interesting. How much solvent did you need for this extraction? And what solvent did you use? Did you recover the solvent by distilling it off
and using it again? Could you provide some more details on how the extraction was done?
Another question I have is what you can do with this orange oil? Does it have some specific interesting chemical properties, which can be used in nice
chemical experiments?
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by woelen  | That's interesting. How much solvent did you need for this extraction? And what solvent did you use? Did you recover the solvent by distilling it off
and using it again? Could you provide some more details on how the extraction was done?
Another question I have is what you can do with this orange oil? Does it have some specific interesting chemical properties, which can be used in nice
chemical experiments? |
I hope he used steam distillation, it's relatively fast, in one morning I processed about 1kg of peels in a few batches, using a solvent would require
lots of it (just to cover the peels) and even more for washings, the beta carotene would also be extracted so a distillation is mandatory. Meh steam
distillation is better
The oil should be mainly limonene, it's used as a solvent
EDIT found this in everyday chemistry hahaha
[Edited on 27-5-2019 by Ubya]
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Yep, this was a steam distillation... actually several. I've never really gotten the hang of the technique so I decided to just do it over and over
for practice. I hadn't thought as far ahead as a use for the orange oil! I might mix some with alcohol and spray it on paper to make scented
stationery for my letter writing. 
In the process I learned that orange oil is produced industrially from the waste from orange juice production by pressing rather than steam
distillation.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Chem Science
Hazard to Others
  
Posts: 122
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Here's some Uranyl Nitrate sample and Thionyl Bromide 
 
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
shouldn't uranyl nitrate be UO2 (NO3)2?
is that an uranium (IV) (with oxygen!?) nitrite?
i think you messed up the formula a bit
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
@Chemscience how much is that?
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
That sample of uranyl nitrate is beautiful.
@Ubya: Uranyl ion has uranium in its +6 oxidation state. The ion is UO2(2+) and the nitrate salt is UO2(NO3)2.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Just enough to convince me to get my own !
Oh please do post a picture in UV light 
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Fresh made Picric acid drying out befor being converted to Ammonium Picrate
https://en.wikipedia.org/wiki/Picric_acid
https://en.wikipedia.org/wiki/Dunnite

Some Iron 2 Sulphate for a 1,4, Dioxan synth I have planed along side some Copper Nitrate
[Edited on 3-6-2019 by XeonTheMGPony]

|
|
|
Chem Science
Hazard to Others
  
Posts: 122
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Ups ... Sorry  Yup i did messed up the formula jej e.. Sorry .. Nex post is on
UV for Herr Haber and the correct formula =) .. btw nice Ferrous Sulphate.I'm very interested in your synthesis of Dioxane, so i'm expectant for the
post Yup i did messed up the formula jej e.. Sorry .. Nex post is on
UV for Herr Haber and the correct formula =) .. btw nice Ferrous Sulphate.I'm very interested in your synthesis of Dioxane, so i'm expectant for the
post 
|
|
|
Chem Science
Hazard to Others
  
Posts: 122
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Here's 20gr of Uranyl Nitrate in UV light 
 
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by woelen  | That sample of uranyl nitrate is beautiful.
@Ubya: Uranyl ion has uranium in its +6 oxidation state. The ion is UO2(2+) and the nitrate salt is UO2(NO3)2. |
yup i know, i was messing around trying to interpret the wrong formula
@chem science: beautiful sample, uranium is one of my favourite elements, if not the most
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
6-Monodeoxy-6-monoazido-beta-cyclodextrin synthesized via refluxingMono-6-O-(p-toluenesulfonyl)-β-cyclodextrin and sodium azide in an aqueous
solution..
Used as synthetic intermediate. The azide is reduced to make 6-Monodeoxy-6-monoamino-beta-cyclodextrin or used in click chemistry to attatch
subsituted functional groups which can be used to bind/trace movement in vivo. One example is performing an amidation reaction between the
amino-cyclodextrin and biotin( biotinylation). When tested on mice, the movement of the cyclodextrin within the cell can be traced by introduction and
binding of streptavidin or avidin. Cyclodextrins can act as "cages" for molecules such as steroids and facilitate drug delivery. B-cyclodextrin itself
is combined with DMSO and a specific therapeutic drug to permit effective drug delivery through cellular walls.
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
some tetraphenylcyclopentadienone made at uni

---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Ubya,
Whoops! You need to change the name of your compound or the structure on the page.
AvB
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
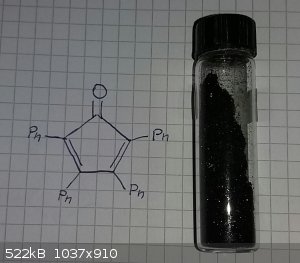
AvB you are totally right, my bad.
ps can't edit my last post (can't edit any of mesagges on this thread but this one, the button is not there, wtf?)
[Edited on 14-6-2019 by Ubya]
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Black organic compound?Weird.
|
|
|
| Pages:
1
2
3
4
5 |