TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Lead electrolysis (To purify lead metal)
Hey SM!
I have some lead I obtained from non spillable lead acid battery, but I have doubts about the purity of it. I need pure lead for precise alloy, I
don't want the impurities to ruin alloys properties, so I need it quite pure. (about 150g, at least)
I don't have an option to heat the lead much more than melting point, and I don't have decent fume hood at the moment. I live in a flat, in well
populated area, so I cant do furnaces and hi temp metallurgy with sulfur and nasty fumes. so I decided to purify it by electrolysis.
I tried to dissolve the lead in acetic acid/peroxide, some of it dissolved but not as much as I would like, and adding peroxide started to get too
expensive, so I stopped when about 70g of Pb was dissolved.
Having some lead acetate solution I figured that I can grow lead crystals by electrolysis, just like with copper, so I attached +4V to lead plate and
-4V to lead mesh and indeed - nice crystals of lead started to grow.
Problems:
1. crystals constantly short out the cell.
2. crystals fall down the solution and touches the anode, theoretically making endless loop.
3. process is ultra slow.
4. not sure if lead is going in the solution from anode
5. there must be easier way to purify lead metal. to at least 99.9%. I could even take just 99%
I read about Betts electrolytic process, but H2SiF6 is nasty acid and I don't know much about it. (I mean how to get to lead fluorosilicate, I have potassium
fluorosilicate, and sodium fluorosilicate, but there is not much information about fluorosilicates.)
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I figure to mention that soluble lead salts are extremely toxic and should not be poured down the drain or on the ground, just as a general FYI to the
masses.
I've used sulfamic acid (available at Home Depot) for lead electrolysis. Lead sulfamate is soluble in water. I don't know, however, if antimony
would plate out with it.
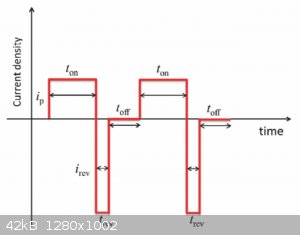
The dendrites can be minimized by pulse-reverse plating. The dendrites form because those are high field intensity areas due to the angular geometry.
Naturally, more metal will tend to plate onto those areas and then problem becomes worse. Breaking up the current flow many times per second refines
the grain. The forward plating current waveform is lower current at a longer time, and the reverse current waveform is a higher current pulse for
less time. The average current should be somewhat in the forward direction, so that metal deposits on the cathode. The lower current forward plating
current plates more evenly due to lower cathode current density, whereas the high peak current of the reverse pulse primarily takes metal off the
sharp edges of the cathode due to the higher current density.
You don't want too high of a current density in either the forward or reverse direction, as this can burn parts of the cathode deposit.
[Edited on 4-11-2019 by WGTR]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by WGTR  | I figure to mention that soluble lead salts are extremely toxic and should not be poured down the drain or on the ground, just as a general FYI to the
masses.
I've used sulfamic acid (available at Home Depot) for lead electrolysis. Lead sulfamate is soluble in water. I don't know, however, if antimony
would plate out with it.
The dendrites can be minimized by pulse-reverse plating. The dendrites form because those are high field intensity areas due to the angular geometry.
Naturally, more metal will tend to plate onto those areas and then problem becomes worse. Breaking up the current flow many times per second refines
the grain. The forward plating current waveform is lower current at a longer time, and the reverse current waveform is a higher current pulse for
less time. The average current should be somewhat in the forward direction, so that metal deposits on the cathode. The lower current forward plating
current plates more evenly due to lower cathode current density, whereas the high peak current of the reverse pulse primarily takes metal off the
sharp edges of the cathode due to the higher current density.
You don't want too high of a current density in either the forward or reverse direction, as this can burn parts of the cathode deposit.
[Edited on 4-11-2019 by WGTR] |
Thanks! 
Yep, I'm familiar with the toxicity.
Ohh, nice, I have sulfamic acid!  Can sulfamic acid solution dissolve lead tho?
(I am trying to limit experiments with lead, so I ask before I try) Can sulfamic acid solution dissolve lead tho?
(I am trying to limit experiments with lead, so I ask before I try)
nice info about pulse-reverse plating, but I am not sure If I could pull off the driver for that. I like electronics, but I'm very basic level, I
imagine it could be done with some mosfets, diodes and 555 timer, but I don't own an oscilloscope, so it would be a shot in the dark with my
knowledge.. 
How about displacement reaction, how pure would be lead grown from solution on more active metal, like copper or zinc?
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
With a lead anode you can dissolve lead electrolytically in sulfamic acid, but it won't react with it directly.
Yes, a 555 timer and a bipolar push-pull MOSFET driver will do the trick, with the addition that the source-to-output for both + and - drivers would
have to be individually current-limited with simple MOSFET current limiters, to control the characteristics of the waveform. The timer would control
the duty cycle, and some dead-time could be built into the driver between forward and reverse pulses.
I couldn't say much about a displacement reaction with more active metals. My first thought is that the reaction would be a mess of finely divided
lead of unknown purity, but I haven't tried it myself.
|
|
|
j_sum1
Administrator
       
Posts: 6225
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
My first thought was a displacement reaction using zinc. I don't know what the purity is like except to say that I have seen some very shiny
(sparkly) crystals produced this way. If light reflection is an indicator of purity then this miggt be a simple route.
One of the main contaminants of Pb (especially from batteries) is Sb. You coUKld ckeck the reduction potentials but I tjink the lead reduces
preferentially.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Thank you!
I'll try a few ideas, see how it goes!
|
|
|
|