Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Sodium metal through ion glass migration
Guys,
I just stumbled on this very odd (but convenient?) way of getting sodium metal from sodium nitrate. This is an excerpt from the Russian counterpart of
this site (traduction through Google, I don’t speak Russian). Has anyone ever attempted this?
| Quote: |
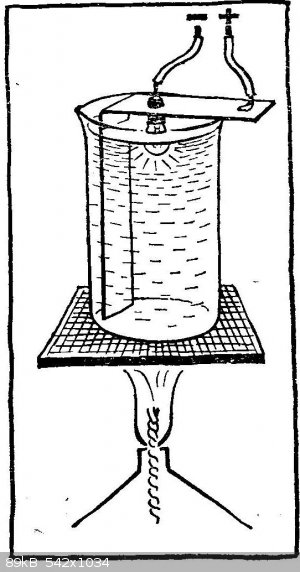
And one more, not quite usual experience with a light bulb, but not from a big one, but from a flashlight. Strengthen it in a strip of tin bent at a
right angle, and insert the strip into a small beaker so that the glass balloon of the bulb is inside the glass and faces the bottom of the bulb.
Connect the light bulb to the battery: connect the protrusion on the base, connect the most extreme part of it to the negative pole, and the tin strip
to the positive pole. Please note: you cannot solder the conductors, because during the experiment the solder may melt. You need to come up with a
mechanical contact or use a cartridge from an old flashlight.
Before starting the experiment, remove the lamp from the glass and pour sodium nitrate into it (potassium nitrate is not suitable in this case; this
will be clarified later). Put the glass on the asbestos mesh or metal plate and heat it on the flame of a gas burner or spirit lamp; dry alcohol is
not very convenient, as it is difficult to regulate the temperature of the melt. Saltpeter melts at 309 ° С, and at 390 ° С it decomposes; here in
this interval and have to maintain the temperature. To do this, change either the size of the flame or the distance to the glass. Make sure that the
melt does not freeze, even from the surface.
Carefully lower the light bulb into the molten saltpeter. Most of the glass bottle must be immersed in the melt, but make sure that the upper part of
the base, to which the conductor is soldered, does not come into contact with the saltpeter, there will be a short circuit. Hold the lit bulb in nitre
for about an hour, then turn off the current, turn off the burner and carefully deliver the bulb. When it cools down, rinse it with water and you will
see that the bulb is covered with a mirror layer from the inside!
We have already said that when heated, charged particles in glass acquire mobility (therefore, the lamp was lit when the tube was heated with a
match). The main actors are sodium ions: already at temperatures above 300 ° C, they become quite mobile. The glass itself remains completely solid.
When you loaded the included light bulb into the melt of nitre, the glass from which the balloon was made turned out to be in an electric field: the
coil is a negative pole, the melt that comes in contact with a strip of tin is positive. The mobile sodium ions began to move in the glass toward the
cathode, i.e. toward the spiral. In other words, they moved to the inner wall of the balloon.
So, mirror coating from inside is sodium? Yes. But how did the ions turn into metal?
The heated metals (including those from which the spiral is made) emit electrons. From the spiral, they hit the inner surface of the glass and
connected there with sodium ions. This formed metallic sodium.
But why is potassium nitrate not suitable for the experience? After all, nitrate does not seem to be involved in the process ... No, it does. When the
sodium ion became a neutral atom, a negatively charged ion hole remained in the glass. Here we need sodium nitrate: from its melt, under the action of
an electric field, sodium ions penetrate into the glass and fill the holes. And potassium ions are about one and a half times more sodium ions, they
will not be able to enter the glass. In potassium nitrate lamp just crack.
Such unusual electrolysis through glass is sometimes used in practice to obtain a layer of very pure sodium, or, more strictly, spectrally pure.
|
[Edited on 16-4-2019 by Keras]
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
This setup can be found in several books. The purpose is to demonstrate that glass becomes a conductor at elevated temeperatures. It works, because
glass bulb is relative thin and is made from proper kind of glass.
Not convenient for making Na on gram-scale.
Слава Україні !
Героям слава !
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Yes, I have done this thing before. You need to use light bulbs that have a vacuum, as opposed to ones filled with an inert gas. Those are not so
common, but 7W small bulbs usually are, as well as the long tubular bulbs. Look for a bulb with a filament that is a straight wire, as opposed to one
that is spiraled into a helix. The bulbs with a straight wire filament have a hard vacuum.
The bulb envelope needs to be soda lime glass as opposed to borosilicate or quartz, as the sodium ion becomes mobile at elevated temperatures in soda
line glass.
In one of the longer glass tubular bulbs, it took the better part of a day to get about a gram of sodium inside. This isn’t much at all, but any
sodium in the bulb is extremely pure, and will remain shiny practically forever. Plus it’s an interesting conversation piece. Of course, towards
the end of my experiment I got the bulb too hot, and the envelop cracked. Molten sodium reacting with molten sodium nitrate makes a lot of nitrogen
gas, which filled the bulb and allowed me to recover some of the sodium. I still, however, have a smaller 7W bulb that is still intact, that
contains sodium.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
The bulb that I cracked was actually damaged by a blob of molten sodium running down the tube to the bottom, at which point the bulb cracked. It's a
challenge to collect the sodium at the base of an upside-down bulb, because, well, gravity and all. If the base can be kept cool enough, then it
should be possible to collect a substantial amount of solid sodium there.
The bulb is essentially working like an old vacuum tube diode. The filament is the hot cathode, and emits electrons. The anode is basically the
glass, if you want to think of it that way. Sodium ions are being reduced by the electrons there inside the glass bulb. I used a graphite anode in
the salt bath to complete the circuit. It's a sacrificial anode, though. It significantly corrodes. I remember seeing puffs of brown nitrogen
oxides coming off the anode (this was performed in a fume hood!)
The cathode is really just a skinny little tungsten wire. You have to power the filament as usual, but keep in mind that whatever current is used to
perform the electrolysis adds to the current that is used to power the filament. Be careful not to overheat the filament and burn it out.
Vacuum tube diodes may have a voltage drop of a few volts, but the light bulb's cathode is very skinny, and relatively far away from the glass
envelope. At least 100V or so is needed before some mA start flowing in the circuit. Once the experiment has run for some time, a brown haze starts
to forms in the cooler areas of the bulb, Eventually, enough thickness builds up that a silver mirror begins to form. After a while little droplets
of sodium will coalesce, and can be seen inside the bulb.
If the temperature of the bulb increases enough such that the vapor pressure of sodium reaches a certain point, there is a sudden "blink!", and a
brilliant sodium flare will light up the room. The sodium vapor is being ionized at that point, and it's the purest yellow color one can imagine.
The current will increase by at least a factor of ten, and electrolysis will proceed that much more quickly at that point. I'd recommend using a
current-limited power supply, otherwise the bulb can pull a very large amount of current, and can explode from over-pressure when the sodium is
ionized like this.
Another option is something that I haven't tried yet, so your mileage may vary. Instead of using a hot cathode, a cold cathode could be used. The
bulb could be filled with a reduced pressure of inert gas, such that the gas would ionize readily. The catch is that it would take much more voltage
to initially fire the tube, maybe several thousand volts to ionize the gas, although the high temperatures involved may decrease that by a large
degree, especially if the work function of the cathode is low. It would essentially work like a glow tube (neon bulb, flash bulb, etc).
One thing that I have tried that is somewhat loosely related to the present topic, is that of making a high temperature sodium "battery". I bought a
box full of soda-lime glass test tubes. Smearing some regular tin-lead solder to the inside of one of these tubes provided a mechanical contact to
the glass (although something like indium would have likely worked much better). Fresh solder tends to bead up into a ball, but if it is oxidized a
bit it becomes possible to smear it around on the glass surface. A wire was attached to the solder for electrical connection, and this wire was
passed through a rubber stopper. I made sure and flushed the tube very thoroughly with nitrogen gas.
I performed a similar electrolysis as before in molten sodium nitrate, for several minutes, to charge the interface between the glass and the solder
with a layer of sodium. Afterwards, the tube was rinsed off in water, and the dried tube was wrapped with copper tape on the outside.
I connected a high impedance voltmeter (around 10 Giga Ohms input impedance) to both the copper tape and the cathode wire, and started heating up the
tube carefully with a heat gun. As the tube heated up, the voltage rose, until it leveled off at around 2.5V, which is about what I would expect from
sodium. Short circuit current, however, was only about 100pA at the most. It was nothing practical, but it was a fun afternoon experiment.
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Thanks a bunch for all the input  I wasn't putting this method forward as a way
to collect a significant amount of sodium, but just as a mere curiosity. Your second experiment sounds very interesting. I wasn't putting this method forward as a way
to collect a significant amount of sodium, but just as a mere curiosity. Your second experiment sounds very interesting.
TBH, I can buy 100g of sodium metal for about 20 bucks, which makes any attempt to obtain sodium metal at home totally pointless to me, given the risk
of working with alcaline metals. But those sorts of experiments are really fun!
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
If sodium is going through the glass into the bulb (or tube),
and no ions are released from the bulb through the glass,
then if you get 'greedy' the bulb must eventually burst ?
P.S. I just looked up some of the properties of sodium,
... provided that you do not boil sodium (b.p. 883oC @ 1 ATM)
its very low vapour pressure means that a sample of sodium in a sealed quartz (or low sodium reactivity) vessel can be safely heated to well beyond
sodium's m.p. (98oC)
... depending upon the inert gas pressure, or lack of it.
So it should be safe to put sealed samples of sodium in boiling water to see liquid sodium ?
(or heat in a flame if you are braver)
I've just disposed of my sodium so I can't try myself.
(and would not have tried even if I still had sodium 
[Edited on 17-4-2019 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Sodium has a lower density in the liquid state, so I guess it depends how full the sealed vessel is.
Ah, here's the post I was looking for:
https://www.sciencemadness.org/whisper/viewthread.php?tid=27...
It contains a picture of my "sodium in a bulb" trick.
|
|
|
yobbo II
National Hazard
   
Posts: 709
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
If you were able to obtain potassium glass would this work for potassium?
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
i must say it's a really cool experiment. tungsten bulbs are banned here but it won't stop me from searching, you sparked my curiosity
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
It is noted that one V. Zworykin said he did this, over 90 years ago.
J Chem Ed 34, 289 (1957)
10.1021/ed034p289
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Below is an old note (1926) with details of the sodium through glass demonstration. It suggests 0.5g is possible using a gas filled 60W bulb.
It does suggest soda glass is required. Unfortunately many tungsten filament type light bulbs use a leaded soda glass which may not be suitable. The
leaded soda glass light bulbs tend to have a gray/black metallics color at the fused joint between the envelope and the stem at the base of the lamp.
Attachment: Na-glass-Burt_rc_1926.pdf (2MB)
This file has been downloaded 339 times
and a note describing a lecture demo
Attachment: sodium-thrughglass-alpern1957.pdf (2.6MB)
This file has been downloaded 306 times
I also suspect that most recent tungsten filament light bulbs use a relatively high gas pressure along with a coiled coil type filament as judged by
the discharge behaviour on an Oudin coil. Even modern microwave ovens tend to use tungsten filament bulbs for in oven illumination and they are
probably still available on ebay.
PS: I just realised car bulbs are still available and a convent size for a small experiment.

These are still available on ebay too. I wounder how much sodium the big one can make per hour.
[
[Edited on 20-4-2019 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Using sodium glass and then using potassium salt is how gorilla glass is made.
Obviously for best results you have to do more than that but that is the basic principle.
|
|
|
|