Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Is there any way to make 3 amino phenol from salicamide?
Is it a possible reaction? I don’t know of any reactions that could do it but I wanted to make sure.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Do you mean 2-aminophenol? I'm guessing you could probably pull that off. 3-aminophenol, though... that's gonna be tough, it violates all of the
regioselectivity rules.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
The Hoffman rearrangement or degradation would work to convert salicamide into 2-aminophenol.
3-aminophenol could be made from phenol without toooo much difficulty - esterify the phenol to phenyl acetate, then nitrate, reduce. The ester group
gives meta- selectivity, as opposed to hydroxyl which gives ortho-, para- selectivity. However, you would want to run the nitration with a substantial
proportion of acetic acid to minimise hydrolysis of the ester during the reaction. Depending on how you do the reduction, the ester may well hydrolyse
in that step; otherwise traditional saponification would work perfectly well.
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Oh dang, could the phenyl acetate be obtained easily by the decarboxcylation of acetyl salicylic Acid?
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
The only issue is how to decarboxylate acetylsalicylic acid without cleaving the ester linkage - in my experience decarboxylations are done under
basic conditions, which are the same for efficient saponifications.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 12thealchemist  | | 3-aminophenol could be made from phenol without toooo much difficulty - esterify the phenol to phenyl acetate, then nitrate, reduce. The ester group
gives meta- selectivity, as opposed to hydroxyl which gives ortho-, para- selectivity. |
OH is the functional
group attached to the ring in pheyl acetate , so it will the one directing the entry of the electrophile in o/p position only.Because of the ester
group,you will end up with predominantly p-nitro phenyl acetate,not m-nitro decarboxylations can be done using just heat too.Also,you need water for ester "hydrolysis",so using solid(fused) NaOH won't cleave
the ester
What you could do:
1.Convert salicylamide to benzoxazolone - http://www.sciencemadness.org/talk/viewthread.php?tid=63327#...
2.Nitrate+ring open it to 2-amino,3-nitro phenol -https://pubs.rsc.org/en/content/articlelanding/1896/ct/ct896...
3.remove the amine by diazotisation+reduction - http://www.orgsyn.org/demo.aspx?prep=cv4p0947
http://orgsyn.org/demo.aspx?prep=CV3P0295
(They have used H2SO4 and HCl to diazotise the amine respectively,but you can use hypophosphorus acid itself as both diazotising
and reducing agent 
[Edited on 18-4-2019 by CuReUS]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
better references -
1.https://onlinelibrary.wiley.com/doi/10.1002/ejoc.201101784
2.https://link.springer.com/article/10.1007/s00706-010-0419-9
3.One pot ring opening,diazotisation and reduction by H3PO2
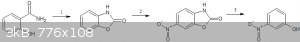
|
|
|
chemplayer...
Hazard to Others
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
Hypophosphorus acid as a one-stop diazotising and reducing agent for the oxazolone ring? That is very interesting - any reference on that would be
much appreciated!
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Very interesting synthesis, and very interesting product too! By ethylation, N,N-diethylaminophenol could be obtained, which is a precursor to many
dyes such as Nile red and Nile blue.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by chemplayer...  | | Hypophosphorus acid as a one-stop diazotising and reducing agent for the oxazolone ring? That is very interesting - any reference on that would be
much appreciated! |
Quote: Originally posted by nlegaux  | | benzoxazolone is dissolved in 100ml 1M HCl(aq) and heated to 80°C with reflux for 1 hour. The solution is allowed to cool to room temperature and
2-aminophenol is filtered. |
Firstly,you will have to open the oxazolone ring using acid.It can be done by
H2PO2 instead of HCl
As for the one stop diazotisation/reduction -https://ia801907.us.archive.org/31/items/MorrisonBoydOrganic... (pg 797 of pdf , 770 in book)
I made a mistake in my route diagram(the product is 3-aminophenol,not 3-nitrophenol).This is the corrected one -
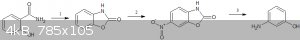
[Edited on 24-4-2019 by CuReUS]
|
|
|