Doktor Klawonn
Harmless

Posts: 29
Registered: 20-11-2010
Location: Europe
Member Is Offline
Mood: No Mood
|
|
How to prepare universal indicator - a recipie
Introduction
Universal indicator is widely used in school and university labs. Surprisingly little information on its composition can be found on the internet
except that its a mixture of indicators. I found one recipie eventually and tested it.
chemicals
- phenolphthalein
- methyl red
- methyl orange
- bromothymol blue
- thymol blue
- ethanole
- water
experimental

0.1 g phenolphthalein, 0.2 g methyl red, 0.3 g methyl orange, 0.4 g bromthymol blue and 0.5 g thymol were weighed and dissolved in a mixture of 250 mL
ethanole and 250 mL water.

The resulting red solution was tested with buffers of different pH. Also, filter paper was soaked in the solution to produce pH indicator paper.
results
The universal indicator on a multi plate at different pH

The universal indicator paper

discussion
This indicator gives a nice color shift through the pH scale. I use it in my chemistry lessons. The colors are slightly different to the universal
indicator bought from merck.
This recipie gives a quite concentrated indicator solution. Some more solvent was necessary to completely dissolve all the indicator dyes.
links
- This procedure as youtube video
- simple experiments with multi plates on acid base reactions
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nice! Do you feel that there is adequate discrimination so that each integral value of pH can be read from say, pH 2 to pH 13?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Doktor Klawonn
Harmless

Posts: 29
Registered: 20-11-2010
Location: Europe
Member Is Offline
Mood: No Mood
|
|
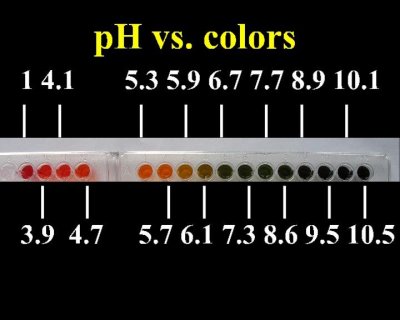
Quote: Originally posted by Magpie  | | Nice! Do you feel that there is adequate discrimination so that each integral value of pH can be read from say, pH 2 to pH 13?
|
It may not be very exact in the higher pH ranges. Near neutral the changes in color are quite pronounced.
|
|
|
bquirky
Hazard to Others
  
Posts: 316
Registered: 22-10-2008
Location: Perth Western Australia
Member Is Offline
Mood: No Mood
|
|
prehehaps a basic question but.
What change happens to the indicator molecules that alters its optical property's ? is it a chemical or physical change ?
|
|
|
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
It gets protonated or deprotonated. It's chemical
|
|
|