Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Tetrahydro Harmaline synthesis idea
I have two questions about a theroretical route to tetrahydroharmaline I have been thinking about.
My route goes something like this:
1. Reissert indole synthesis but instead of diethyl oxalate, dimethyl methylmalonic acid is used, and instead of 2-nitrotoulene, 4-MeO-2-nitro toulene
is used
2. The carboxcylic acid group is converted to an amide with ethanolamide through a similar prodcedure as :
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Amides/Synthesis_of_Amides/Making_Amides_from_Carbo
xylic_Acids
3. A Hoffman rearrangement is done on the compound
4. The OH group is converted to Br using HBr in the prescense of H2SO4
My questions are, can the indole synthesis even be performed, and how can I close the ring?
[Edited on 4/25/2019 by Jackson]
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
Please give more information, I have no idea from what you start aswell what specific conditions you use.
I had a super quick look into "Indole Ring Synthesis by Gordon W. Gribble pp 332-337" for the Reissert indole synth and didn´t have a clue due to
missing information. I did not put any afford into though.
Edit: I´m into the Fischer atm so don´t blame on me please
[Edited on 25-4-2019 by DrScrabs]
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
I was gonna draw this out for you but then i realized that it straight up won't work at all.
Step 1: The reissert indole synthesis calls for dialkyl oxylate not dialkyl malonate, this would leave you not with an indole ring but instead some
kind of dihydro pyradine ring, thats assuming the mechanism would even allow for an extra carbon in between the 2 carbonyls.
Step 2: your link doesn't work.
Step 3: also cannot work as a hoffman rearrangement cannot work on a N substituted amide, because the reaction proceeds via an isocyanate which cannot
have any substituents on the nitrogen.
Step 4: might work but under those those condition you run a small risk of cleaving the methoxide (though this is unlikely).
There are plenty of other ways to synthesize harmaline however your gonna run into the trouble of somehow reducing the imine of the ring substituted
beta carboline to your tetrahydroharmaline without also reducing the alkene on the indole ring.
Perhaps there is a methods out there that doesn't involve cyclization via amination.
https://en.wikipedia.org/wiki/Harmine#/media/File:Harmine_Bi...
Sufficiently advanced science is indistinguishable from madness.
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Thank you for the responses. They have been very helpful 
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
Step 1 actually makes sense, but Hofmann rearrangement works only on amides of ammonia.
On a second look, you would have to use 1-(3-something-propyl),2-nitro,4-methoxy benzene as a starting material then close the ring at the end
somehow.
[Edited on 26-4-2019 by TGSpecialist1]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Assured Fish  | | you are gonna run into the trouble of somehow reducing the imine of the ring substituted beta carboline to your tetrahydroharmaline without also
reducing the alkene on the indole ring |
Its not that difficult since the indole double bond is resonace
stabilised,so much harder to reduce -https://youtu.be/Hif_SnVgkJA?t=345
https://commons.wikimedia.org/wiki/File:Reissert_Indole_Synt...
In the 3rd step,I think the elimination of the ethoxide depends on the COOEt next to it,which isnt' there in diethyl molanate
Also,the double bond formed after the CH3 of nitrotoluene reacts with diethyl oxalate is stabilised by conjugating with the COOEt,which
won't happen in malonate -http://www.orgsyn.org/demo.aspx?prep=cv5p0567
So based on these facts,oxalates can't be substituted by malonates in the reissert indole synthesis
| Quote: | | On a second look, you would have to use 1-(3-something-propyl),2-nitro,4-methoxy benzene as a starting material |
changing the no of carbons on the benzene ring won't contribute to the indole ring
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
Elimination of alcohol happens during aqueous workup, reaction with dimethyl methylmalonate produces intermediate in picture and these extra carbons
are for the 6-carbon nonaromatic ring: https://en.wikipedia.org/wiki/Tetrahydroharmine
[file]75156[/file]
[Edited on 29-4-2019 by TGSpecialist1]
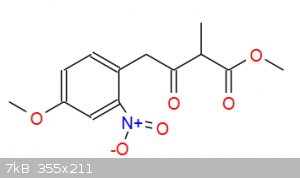
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
You could start with compound pictured, and at the end form the amide then reduce it with LiAlH4/LiBH4.
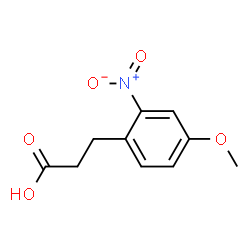
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
If you legitimately want to make this tetrahydro harmaline material then there is no point trying to reinvent the wheel.
https://en.wikipedia.org/wiki/Pictet%E2%80%93Spengler_reacti...
Sufficiently advanced science is indistinguishable from madness.
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
Yes, but the right precursors are hard to get.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Even if it did produce that,your
methyl group would still be in the wrong place | Quote: | | and these extra carbons are for the 6-carbon nonaromatic ring |
I count only 2 carbons
What happened to "1-(3-something-propyl),2-nitro,4-methoxy benzene"
? | Quote: | | and at the end form the amide then reduce it with LiAlH4/LiBH4. |
could you draw out the entire route ?
doesn't mean they can't be made 
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
It's this, I just figured out that "something" group could be carboxylic acid. 
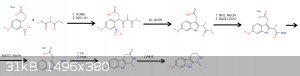
Some steps could be done differently or in different order.
edit: Now that I thoroughly looked at it, I think that a Reissert reaction may not be possible at all with a malonate ester, it will form a salt which
will probably not react. Methyl 2-methyl-3,3-dimethoxypropionate could be used instead or even better, ethyl D-alaninate, it will make the reaction
stereospecific for active isomer and easier.
[Edited on 1-5-2019 by TGSpecialist1]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Not bad,the simplicity and straightforwardness
of the route(if the first step worked) is very nice.I also like the way you introduced the N from the C-2 carbon rather than from the more obvious and
common C-3 carbon of the indole(i.e via tryptamine) 
| Quote: | | I think that a Reissert reaction may not be possible at all with a malonate ester, it will form a salt which will probably not react.
|
why do you think malonate will form a salt when oxalate won't ? | Quote: | | Methyl 2-methyl-3,3-dimethoxypropionate could be used instead or even better, ethyl D-alaninate, it will make the reaction stereospecific for active
isomer and easier. |
please draw it out 
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
The precursor, 3(2-nitro-4-Methoxyphenyl)propionic acid could be made by a heck reaction between p-chloro-methoxyphenol and acrylic acid followed by a
nitration
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
I mean, the hydrogens on the middle carbon of malonate ester are relatively acidic.
| Quote: | please draw it out  |
For D-alaninate (amine may have to be protected) the reaction is the same, just skip steps 3 and 4. For 2-methyl-3,3-dimethoxypropionate the reaction
is also the same but you also have to hydrolyse the dimethyl acetal then oxidize the aldehyde to carboxylic acid.
Quote: Originally posted by Jackson  | | The precursor, 3(2-nitro-4-Methoxyphenyl)propionic acid could be made by a heck reaction between p-chloro-methoxyphenol and acrylic acid followed by a
nitration |
The methoxy group is more strongly o-directing than alkyl and Heck reaction would give a cinnamic acid.
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
A better and easier route may be to do an Aza Diels-Alder reaction of a 6-MeO indole with N-Vinylmethanimine which could be made from Vinylamine and
Formaldehyde in situ (i think).
Edit: Oops. This would only make a beta carboline, not the product I am looking for.
To get a Harmaline I think you would have to use (1E)-N-Vinylethanimine If that reaction even works.
[Edited on 5/3/2019 by Jackson]
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
Also, Diels-Alder doesn't work with aromatic double bonds (I think).
edit: And even if it would, half of the diene would attach upside down.
[Edited on 3-5-2019 by TGSpecialist1]
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Oh okay. Thanks 
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
I don't think you can
call that a salt,because it will only exist as long as there is no H+ ion nearby the better and easier route is to
react 6-MeO tryptamine with acetaldehyde, like fish said 
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
If it has a cation and anion it's a salt.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The oxalate is necessary for the Reissert synthesis to proceed. Other esters do not work. In particular, diethyl malonate and nitrotoluene will simply
yield the malonate ester carbanion.
If oxalate were not necessary, it would never be used! People would just use ethyl formate and make an indole with no 2-carboxylic acid.
A particular example of this difficulty occurs with the synthesis of 4-bromoindole. While the Reissert synthesis proceeds as usual on
6-bromo-2-nitrotoluene to yield 4-bromoindole-2-carboxyl-ethyl, the decarboxylation of 4-bromo-indole-2-carboxylate is nearly impossible; it
decomposes before thermal decarboxylation. If other esters could be used, this would not be a problem. DMF dimethyl acetal is used instead as a more
reactive formyl synthon.
[Edited on 3-5-2019 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
But why it does have to be an oxalate ester?
|
|
|
Tkuze
Hazard to Others
  
Posts: 108
Registered: 8-5-2019
Member Is Offline
|
|
Send me a U2U. Im very interesrted in your progress and results qnd can carry out a reaction for you if you dont have the gladsware, nitrogen tank,
equipment, or reagents. Let me know what i can do bud. Lets publish a paper
|
|
|