Phthalide and reductions of anhydride to lactone
I thought that the topic of anhydride reduction to lactone is interesting enough on it's own, so it can be separated from https://www.sciencemadness.org/whisper/post.php?action=reply... into a new thread.
Quote: Originally posted by oberkarteufel  | About the pathaway starting from succinic acid:
Ha, no further than 3 days ago I found in my Vogel a synthesis of (IIRC) homophthalic acid, with an intheresting intermediate - phthalide. It's
synthesis looks like this:
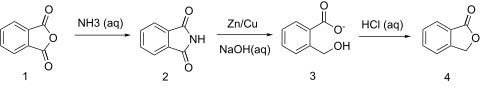
So I wondered, if such reaction is possible with succinic acid/anhydride/imide/choose your own starting point. |
Quote: Originally posted by advanced warning  |
That's a very interesting synthesis you've posted. Apparently, yes, you can do the same thing with succinic acid. The reaction with ammonia, at least,
to form succinimide.
https://thosci.com/synthesis-of-suiccinimide/
Unrelated to the topic at hand, but from there, you can brominate the succinimide in an aqueous solution with NaOH to yield NBS. Potentially a very
useful pathway, from OTC chemicals.
I was interested more in the reduction of succinic acid to yield THF, but if the pathway you've posted holds up for succinic acid, it may be an easily
accessible option for GBL.
edit:
I can't imagine it would be all that hard to construct a suitable pressure vessel for containing such a reaction. The biggest issue would be
pressurizing a hydrogen atmosphere. I don't know how you could do this outside of an external tank. Perhaps you could generate hydrogen gas in some
sort of sealed container (like the NaOH + Al method). I'm imagining a pressure cooker type system with an attached pressure gauge. You could attach
some sort of ball valve and gas line to the reaction vessel, and pressurize it.
You'd still need some way to flush the system with nitrogen, or risk turning your steel reaction vessel into an IED. Nitrogen tanks are fairly cheap I
imagine. It seems fundamentally simple though. So long as you take appropriate precautions, you could easily hydrogenate effectively anything,
forever, in a batch-wise process. So long as you have a catalyst, of course.
Am I wrong in this assumption?
[Edited on 9-5-2019 by advanced warning] |
Quote: Originally posted by advanced warning  | This patent:
https://patents.google.com/patent/US2919282A/en
reveals that phthalimide can be reduced with various metals, such as copper, aluminum, and zinc with an alkali hydroxide. When reacted with acid, this
yields phthalide. The yields are reported to be around 90-95%.
This should be analogous to succinimide to GBL. The literature is lacking, but correct me if I'm wrong on this assumption.
Of course, this reaction may not be necessarily more practical than GABA diazotization, as copious amounts of NH3 gas would need to be safely vented.
|
And to add some information - I translated 2 descriptions of phthalimide preparations. One was based on 4th edition of Vogel, so in this case it was
translated back into English again 
Quote: Originally posted by Vogel  | The starting compound is phthalic anhydride and it's transformed into phthalimide by acting with aqueous ammonia, or, more conveniently, urea.
Reduction of phthalimide with mixture of zinc and copper in presence of base is the most convenient laborathory method of phthalide synthesis; however
it can also be obtained by direct reduction of phthalic anhydride.(...)
Phthalide. 90g (1,37mol) of high quality zinc powder is mixed into a thick paste with a solution of 0,5g crystallized copper (II) sulfate in 20ml of
water (CuSO4 activates the zinc) in a 1L round bottom flask with 3 necks and then 165ml 20% NaOH solution is added. Flask is cooled in an ice bath to
5°C and with mechanic stirring 73.5g (0,5mol) of phthalimide is added with small portions, so the temperature doesn't rise above 8°C (addition takes
up to 30min.). Reactants are stirred for another 30min., diluted with 200ml of water and heated on a water bath until ammonia evolution ceases (about
3h). Solution is concentrated by vacuum distillation to 200ml and is filtrated. The filtrate is cooled in ice and acidified with concentrated
hydrochloric acid (about 75ml) against Congo paper. Most of phthalide separates as an oil, but in order to complete the lactonization of
hydroxymethylbenzoic acid the mixture is heated to boiling for 1h and then, still hot, is poured into the beaker. Separated oil solidifies after
cooling into hard, red-brown mass. Mixture is left in a fridge until the next day and then is vacuum filtered. Raw phthalide contains lots of NaCl; it
is recrystallized in portions of 10g in 750ml of water; mother liquor after first crystallization is used for the nex crystallization. Each portion is
filtered hot, cooled in ice under 5°C, filtered again and washed with small portions of ice-cold water. Product is dried in the air. Phthalide yield
(m.p. 72-73°C) is 47g (70%). |
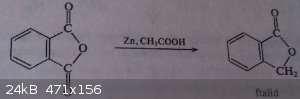
Quote: Originally posted by Some yet another handbook of preparative chemistry  | Phthalide
Reagents:
Phthalic anhydride 80g
Acetic acid 80% 310g
Hydrochloric acid (d 1170-1180g/dm3) 250g
Zinc powder 115g
Sodium carbonate, saturated solution 600cm3
Into a 2L 3-necked flask with a reflux condenser, stirrer and a termometer is put 310g of 80% acetic acid, 250g hydrochloric acid and 80g of phthalic
anhydride. Then, with stirring and heating slowly to 85°C 60g of zinc powder is added in portions. Temperature 80-90°C is kept and the next portion
of 55g zinc powder is added and the heating of reaction mixture is continued in this temp. for 10h, with constant stirring. Reaction mixture is then
diluted with 500cm3 of water and small amounts of unreacted zinc is filtered off. Filtrate's pH is adjusted to 4-4,5 with a saturated solution of
sodium carbonate (500-600cm3) and the filtrate is left in a fridge overnight. After filtration and washing with water the precipitate is dried in a
vacuum desiccator over anhydrous calcium chloride.
Yield: 40-45g of phthalide. Product can be recrystallized in water (1:70), and mother liquors can be used to dissolve the next portion of raw
phthalimide.
Properties: Melting point of pure compound 70-72°C
|
|