Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Minimum charge diameter with sensitized ammonium nitrate?
Using non explosive sensitizer(s) (no NG, PETN, nitromethane, TATP etc.) and AN, what is the minimum charge Dia. that can be used with reliable
propagation? Assuming good tamping and containment, as in a bore hole-
|
|
|
mabuse_
Hazard to Self
 
Posts: 56
Registered: 3-6-2010
Member Is Offline
Mood: No Mood
|
|
This might be interesting for you:
http://www.wydawnictwa.ipo.waw.pl/cejem/vol-6-1-2009/Zygmunt...
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Laboratory curiosity or practical application ?
By including crystallization modifiers used with melts rapidly flash cooled and granulated in the anhydrous condition, critical diameter and cap
sensitivity below 1/2 inch has been reported. See GB1014071, page 13 Example 2, describes a melt composition of 79% NH4NO3, 20% urea, 1%
methylcellulose, granulated on cooling and packed was cap sensitive at 3/8" in copper tubes. What would be the phase stability and storability for
such a composition is unreported, but it is probably poor. Using fuels which form adducts like hexamine or glycine can help keep the critical
diameter low while keeping the density up, and aluminum flake can help also. Solid or plastic emulsions would probably have the best storability and
reliability. There are an assortment of patent and proprietary compositions where various minor ingredients have been used as refinements.
Some of them are cap sensitive at 2" or even less. The crystal size and phase of the NH4NO3 is a definitive factor.
Very specific crystalline forms of pure NH4NO3 (for the interval they last) are cap sensitve, without any sensitizer whatsoever. But this is a
laboratory curiosity without some way to stabilize the NH4NO3 against its usual behavior of changing crystalline form with temperature and humidity
changes during ordinary storage.
Attachment: US4746380 Glycine NH4NO3.pdf (145kB)
This file has been downloaded 1051 times
[Edited on 9-12-2010 by Rosco Bodine]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
I was thinking in terms of diameters workable in practical applications rather than laboratory curiosities- But lab curios are instructive...
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
IIRC one of the better simple 2 component oxidizer - fuel systems was acetylenedicarboxylic acid with ammonium nitrate, and I think it was a melt cast
that was cap sensitive
having sufficient energy and velocity to make it useful as a booster for blasting agents. Emulsions are a lot cheaper though. There have been some
foamed melt casts using urea. Some of these mixtures may actually react chemically to form in situ separate energetic compounds which sensitize the
mixtures.
The acetylenedicarboxylic acid sensitized oxidizer actually used a mixture of different oxidizers, probably for lowering the melting point but likely
also for phase stabilizing the NH4NO3 component of the mixture. See US4689096 .
[Edited on 9-12-2010 by Rosco Bodine]
|
|
|
grndpndr
National Hazard
   
Posts: 508
Registered: 9-7-2006
Member Is Offline
Mood: No Mood
|
|
Im assuming, maybe wrongly your referring to ANFO and the smallest diam borehole practicable.
I did a quick search google (your job) and came up with at least 20 related pages of info concerning anfo and borehole diameter ,density etc.FWIW one
of the sources reccomended a 3in min borehole w/anfo another 5ml larger at 80mm.I quit after seeing 20pages of related material free for the effort of
reading it.Anfo must be one of the most thoroughly researched explosives out there just take advantage off the published literature
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
grndpndr, wouldn't it have been quicker to type UTFSE? (I did, filtering that
mass of info on an iPhone in the wilderness wasn't going well) (I did, filtering that
mass of info on an iPhone in the wilderness wasn't going well)
3" isn't going to do it. 1/2" to 1" Dia. is more useful. The link to ammonals and Aluminim containing emulsions provided by mabuse_ covers the range
of diameters I am interested in. More specific application information will probably get this thread shut down.
I'm curious also as to how those water emulsions behave at low temperatures. Temperatures are running below zero F here lately.
(edit) looks like they don't work at low temps without additional sensitizers such as amine nitrates.
[Edited on 14-12-2010 by Bert]
|
|
|
grndpndr
National Hazard
   
Posts: 508
Registered: 9-7-2006
Member Is Offline
Mood: No Mood
|
|
Why did you withold pertinent information!1.5 in borehole?!That
narrows down the AN sensitizers some wouldnt you say
And why Didnt/dont? you UTFSE!
[Edited on 15-12-2010 by grndpndr]
[Edited on 15-12-2010 by grndpndr]
[Edited on 15-12-2010 by grndpndr]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
A test shows that Tannerite will propagate in a lightly confined 1/2" Dia. column- This is the results of about 6 Oz. loosely loaded in a 4 foot
length of 1/2" CPVC plastic water pipe. witness plate is 1/2" OSB board. This is a test for a movie special effect shot.
Heavy grain det cord is more appropriate and dependable for such effects- Any packing or settling of the charge results in a failure to propagate.


|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Interesting AN-CN Double Salt mixtures
Here is another method of producing low critical diameter and cap sensitivity even for not fully anhydrous AN compositions. See US4084995 attached
Attachment: US4084995_CAN_cap_sensitive_mixture.pdf (273kB)
This file has been downloaded 652 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
This must have been covered here somewhere
Blaster Training Manual / one continuous url
http://cdn.preterhuman.net/texts/terrorism_and_pyrotechnics/explosives/Demolition_Blasting_Sabotage/Blasters%20Training%20Manual%20(Farmers,%20Rancher
s,%20Prospectors%20&%20Engineers)%20By%20R%20K%20House.pdf
From bottom pdf page 103 , or just search " less than "
" ANFO mixtures are generally suitable only for heavy loadings in large diameter
shotholes, because their explosive reactions become more efficient as the
column diameter increases and the quantity of the ANFO is increased. Blasting
agents are seldom used in shotholes less than 4 inches in diameter, and are
not reliable or efficient in small shots."
Blaster Certification Training Manual
http://deq.mt.gov/CoalUranium/blaster/BlasterTrainingManual....
See top of pdf page 38 ( document page 26 ) " Charge Diameter "
Explosives & Blasting Procedures Manual
https://archive.org/details/explosivesblasti00dick
From there select HTTPS from left column to access the direct file downloads here _
https://ia701206.us.archive.org/4/items/explosivesblasti00di...
From the .djvu format , see page 66 ( document page 52 ) bottom
" With few exceptions, economics and efficiency favor the
use of bulk loading in blasthole diameters larger than 4 in. The
products are cheaper, loading is faster, and the well-coupled
bulk charge gives better blasting efficiency."
https://ia701206.us.archive.org/4/items/explosivesblasti00di...
Complete Blaster's Guide
http://www.austinpowder.com/BlastersGuide/docs/0-%20Complete...
Attachment: NH4NO3 & Mixtures Detonability .pdf (263kB)
This file has been downloaded 460 times
Attachment: NH4NO3 Rates of Reaction in detonation.pdf (696kB)
This file has been downloaded 454 times
 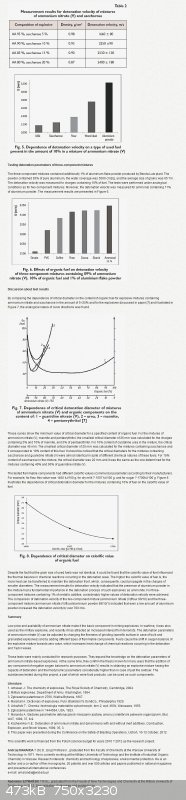
________________________________________________________________________
Rosco Bodine has posted for you the lower bleeding edge of investigation into ' minimal ' diameter.
I can suggest experimentation using organic acids as these sensitize AN more than aliphatic or
carbohydrate fuels. Try acetic , and carboxylic compounds.
On my own I have been musing at in situo chemistry as done with Astrolytes.
NH4NO3 + N2H4 => N2H4•HNO3 + NH3
Add mixing Sodium Nitrite to a saturated water solution of AN yields Ammonium Nitrite
NH4NO3 + NaNO2 => NH4NO2 + NaNO3
NaNO2 - Solubility in Water: 46% @ 20 ºC
NH4NO3 - Solubility is twice as much higher
I can find no literature on this , just this related patent US2285843
Likely because it's unstable and quickly hydrolyses. Non aqueous solvation does not appear fruitful since
the amounts dissolved are low.
NH4NO3 - 100g Isopropanol or Methanol dissolve 17g @ 20 ºC
NaNO3 - 100g Methanol dissolves 16.7g @ 30 ºC
NaNO2 - 100g Methanol dissolves 4.4 g @ 25 ºC
NaNO2 - 100g Ethylene Glycol dissolves 16.7 g @ 25 ºC
[Edited on 11-9-2014 by franklyn]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by Rosco Bodine  | | Here is another method of producing low critical diameter and cap sensitivity even for not fully anhydrous AN compositions. See US4084995 attached
|
Curious to know of any low temperature related sensitivity decrease to these mixtures, particularly those without nitrotoluenes- They don't specify
the testing conditions... Even Tannerite seems to get a bit sluggish at -10 F. Several of the other mixtures I looked at when this was a new thread
were not all weather performers either.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1339
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
AN diameter
I can do tests in diameter 12.5 mm. (Patent US3247033 A) describes VoD 3100 m / s in 0,5 inch. This composition is tested on average 19.5 mm. A work
from No.8. 1 meter long plastic tube? No problem. The mixture must be dry. The density of 1 to 1.2 g / cc. Wooden board? How thick? Or any other
source material?.....
LL
|
|
|