| Pages:
1
2 |
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Sulphonation of chlorophenol
I am trying to prepare an azoic dye intermediate: 2-amino-4-chlorophenol-6-sulphonic acid. I have been unable to find a synthesis of this important
dye intermediate so I decided to try the following route from 4-chlorophenol which I already have.
4-chlorophenol ---via sulphonation---> 4-chlorophenol-2 and possibly 2,6-disulphonic acids ---nitration---> 2-nitro-4-chlorophenol-6-sulphonic
acid ---reduction---> desired product
The problem is that the 4-chlorophenol seem very resistent to sulphonation. I would expect it to be less reactive than phenol but the compound is very
unreactive inspite of the fact that it nitrates fairly easily to 2,6-dinitro-4-chlorophenol.
Does anyone have any experience/reference to such sulphonations? I have an entire book on naphthalene derivatives but I can't find a similar book
dealing with the basic benzene derivatives. Alternatively a reference to chlorophenol chemistry, even an old or German one would be a great help.
Attachment: chlorophenolsulphonic acid.emf (19kB)
This file has been downloaded 500 times
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Arene Chemistry, edited By Jacques Mortier, Wiley 2016 maybe?
Also what did you use to sulphonate? Sulphuric acid?
[Edited on 20-5-2019 by Keras]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Yes I am using conc sulphuric acid at about 150-180 C, its been cooking now for 2 hours. The chlorophenol is now disssolving slowly.
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Boffis  | | Yes I am using conc sulphuric acid at about 150-180 C, its been cooking now for 2 hours. The chlorophenol is now disssolving slowly.
|
I'd be concerned that at that temperature and concentration you'd get the sulphuric acid to react with the OH group and get some sort of ester. Cl -
Ph - O - SO2(OH). But maybe I'm way off base.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Found it!! Organic Chlorine Compounds by Huntress 1948; Its nice to have some of these really old chemistry books on ones shelf sometimes.
It the bible of organic chlorine chemistry to that date!
Got some refs: Petersen et al., Annalen 157, p128-147 [1871] and Guantlett, JCS v127, p2745-2746 [1925]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
you could try using stronger sulphonating agents like oleum or chlorosulphonic acid
the other thing you could do is
1.sulphonate phenol - https://pubs.rsc.org/en/content/articlelanding/1985/p2/p2985...
2.chlorinate o-phenol sulphonic acid using bleach -https://pubs.acs.org/doi/abs/10.1021/ed025p514?journalCode=j...
3.Nitrate and reduce to 2-amino-4-chlorophenol-6-sulphonic acid
| Quote: | | I am trying to prepare an azoic dye intermediate.. |
could you tell what dye you want to make ?
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
My textbook suggests that sulphonation is a slow reaction anyway, because it relies on the auto-protonation of a sulphuric acid molecule. Nitration is
much quicker because if you add sulphuric acid to nitric acid, the former, which is more powerful, protonates the latter, which releases a water
molecule and the NO2+ cation which proceeds to attacking benzene.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@CuReUS, yep looking at the references both oleum (20%) and chlorosulphonic acid have been used in the past. Its interesting to note that phenol
reacts much more quickly. I have found in the past that conc sulphuric acid does most of the things that oleum does, it just take a lot longer as
Keras points out sulphonation is generally slow.
The 4-chlorophenol dissolved completely after about 4 hours.

The chlorination of o-phenolsulphonic acid sounds interesting but how do you selectively o sulphonate phenol? The alternative is general sulphonation
and then separation which is a pain but doable.
This azoic intermediated when diazotized couples with resorcinol (Lumogallion reagent for In and Ga), barbituric acid (Lumomagneson, reagent for Mg)
and chromotropic acid etc
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Boffis  |
The chlorination of o-phenolsulphonic acid sounds interesting but how do you selectively o sulphonate phenol? The alternative is general sulphonation
and then separation which is a pain but doable. |
The -OH group is highly nucleophilic, it will favour the addition of electrophiles such as HSO3 in o- and p- positions. The yield should be
⅔ o- ⅓ p- just for geometrical reasons. Both isomers should have very different properties and b.p. so separating them by fractional distillation
shouldn’t be much of a hassle.
[Edited on 21-5-2019 by Keras]
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
"⅔ o- ⅓ p- just for geometrical reasons"
I'm almost sure that the ratio will be different, more p, less o, - just for geometrical reasons. :-)
I mean: there is the OH group at position 1 and it requires some space. Not too much, but some. The big and bulky sulfonating species prefers "empty"
space around it so statistically somewhat more than 33% p and consequently less than 67% o isomers will form.
I'd be really surprised if the ratio would be what you stated.
(Although chemistry left me surprised numerous times, especially when it was about preparations.)
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Pumukli  | "⅔ o- ⅓ p- just for geometrical reasons"
I'm almost sure that the ratio will be different, more p, less o, - just for geometrical reasons. :-)
I mean: there is the OH group at position 1 and it requires some space. Not too much, but some. The big and bulky sulfonating species prefers "empty"
space around it so statistically somewhat more than 33% p and consequently less than 67% o isomers will form.
|
Okay, there might be steric hinderance, but at least when you do the nitration of phenol, you get 2/3 o-, 1/3 p-.
But you're right. It seems temperature is key here. At low temp (r.t. or below, such as what's need for single nitration), ortho- form is preferred,
but as the temp increases, more and more para- is formed since this is the thermodynamically preferred form.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
You can't. You have to sulphonate and separate the isomers.But
you get a decent yield of the o-product (49%) You
are right,but barely.The ratio is almost 1:1 - o-49%:p-51% (see the paper i linked above) | Quote: | | I mean: there is the OH group at position 1 and it requires some space. Not too much, but some. The big and bulky sulfonating species prefers "empty"
space around it so statistically somewhat more than 33% p and consequently less than 67% o isomers will form. |
steric hindrance isn't the only factor determining regioselectivity.Other factors are:
1.diffusion control of reaction rate
2.Hydrogen bonding
3.Complexation of substrate and electrophile ( reason for high o-yield in phenol) Quote: Originally posted by Keras  | when you do the nitration of phenol, you get 2/3 o-, 1/3 p-.
It seems temperature is key here. At low temp (r.t. or below, such as what's need for single nitration), ortho- form is preferred, but as the temp
increases, more and more para- is formed since this is the thermodynamically preferred form. |
the
concentration of the sulphuric acid used in the nitration determines the o/p ratio.The o/p ratio of phenol varies from 1.9 to 0.9 over the acid range
of 61 to 83% H2SO4
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
That’s what the article claims from a reference, but the authors' own findings indicate that the yield is 50/50 over 73 to 90% concentration range.
Also the reaction here is made at r.t. Also the authors warn that with concentrated (> 88%) sulphuric acid, the reaction produces the 2,4-product.
I also found this, might be interesting.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Actually when I looked through my stash of chemicals I discovered that I have already done the sulphonation of phenol and have about 150g of barium
p-phenolsulphonate and about 100g each of sodium and barium o-phenolsulphate from many years ago. I had completely forgotten about them. However,
since I already have 4-chlorophenol this seem like the obvious starting material.
I cooked the sulphuric acid/chlorophenol mixture for 4 hours at 160 C in the end and got a light brown homogenous solution which I then nitrated
without isolation by adding 6.51g of sodium nitrate (1 molar equivalence) in small amounts. The reaction proved to be very slow so I warmed it gently
and a very quiet reaction set in with the separation of a pale yellowish brown powdery substance. After warming (to about 60 C) for an hour I drowned
the slurry into 200ml of cold water. I expected to get a clear solution apart from perhaps a little un-sulphonated 4-chlorophenol and
nitro-4-chlorophenol. In fact I got a significant amount of yellow-brown insoluble material. It is partly soluble in dilute alkali giving a faintly
brownish solution that gives an amazingly intense violet with tap water even at insanely low concentrations. It doesn't give a distinctive colour with
ferric chloride even when carefully neutralised. Mmmmm
The solid is drying and the light orange filtrate is about to be neutralised and evaporated down to see what I can find in it.
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Boffis  |
The solid is drying and the light orange filtrate is about to be neutralised and evaporated down to see what I can find in it. |
Please keep us posted!
EDIT: To be honest, I think you should have filtered and purified your product before proceeding to nitrating it. I'm sure you ended up with a ton of
side products…
[Edited on 21-5-2019 by Keras]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Keras. Yep, as I start to isolate products its quite clear that I have a real cocktail. The insoluble solid when dried weighed 6.51g but is clearly a
mixture under the microscope. Recrystallizing it from isopropanol generates beautiful almost colourless six-sided monoclinic plates several mm across.
On further evaporation tiny yellow granules form. The former I suspect will turn out to be un-reacted 4-chlorophenol but the latter are less clear. I
thought the compound may be 4-chloro-2-nitrophenol but this is clearly not the case as I have this compound prepared from p-dichlorobenzene via
nitration to 2-nitro-p-dichlorobenzene then via pressure-cooking with NaOH the Cl in the 1 position is replaced to give the appropriate phenol
derivative.
This latter route makes me wonder if the route to my desired compound into via 2-nitro-p-dichlorobenzene to 2-nitro-4-chlorophenol then via
sulphonation to the 6-sulphonic acid and finally reduction the the amine. I suspect that the sulphantion of the nitrochlorophenol will be slow and
difficult without oleum too.
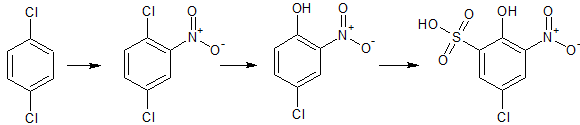
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
I’m surprised you didn't start from phenol, but maybe that makes sense since chlorinating phenol will give you a mix of p-choloro and
o-chlorophenol, I suppose.
Why don't you run a TLC test to find out how many different compounds you have in your mix?
Also, maybe this could be useful to you? It's an excerpt from Vogel's Textbook of Practical Organic Chemistry.
[Edited on 22-5-2019 by Keras]
Attachment: p-toluenesulfonic_acid.pdf (344kB)
This file has been downloaded 345 times
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have encountered a slight problem. Chlorophenol is supposed to have a low melting point and faint unpleasant smell. My 4-chlorophenol has almost no
smell, perhaps faintly clinical. I have also just done a melting point test and it didn't melt below 62 C at which point I gave up; the published
values range up to 42 C. So I think I have a problem with my starting material.
I can prepare 4-chlorophenol from phenol and sulphuryl chloride and I am currently checking out the detail of this prep.
Also reading some of the old German literature it appears that when most chlorophenol sulphonic acids (both 2 and 4 Chlorophenol) are nitrated the
sulphonic acid group is replaced by a nitro group giving one or more nitro-chlorophenols. So this does seem to suggest that I will have to go via
sulphonation of the 2-nitrochlorophenol after all.
Some years ago I prepared heaps of 2-nitro-1,4-dichlorobenzene (>200g) as I had lots of p-dichlorobenzene and this seemed to be the only reaction
it would undergo. The problem with converting it to 2-nitro-4-chlorophenol is that it needs an autoclave that with operate at 3-5 Bar AND has internal
stirring, my autoclave is rated at 90 bar but doesn't have a facility for stirring. Do you think that I could convert this compound to the appropriate
2-nitro-4-chloroaniline by fusing with urea in a similar method to producing trinitroaniline from from picryl chloride? The aniline would then be
converted into a diazonium compound and hydrolysed to give the appropriate phenol.
In the mean time I have isolated beautiful yellow plates from my crude nitration product, god only knows what it is. I will report back later on
progress and also further investigations into my "4-chlorophenol".
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
I'd try. :-) I don't know how the nucleophilicity of NH3 compares to the NH2's in urea though. But amines are stronger nucleophiles than water in
general and maybe at the temperature of the melt it could be used for advantege.
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Boffis  | I can prepare 4-chlorophenol from phenol and sulphuryl chloride and I am currently checking out the detail of this prep.
|
Prepchem has this.
Quote: Originally posted by Boffis  | | Some years ago I prepared heaps of 2-nitro-1,4-dichlorobenzene (>200g) as I had lots of p-dichlorobenzene and this seemed to be the only reaction
it would undergo. The problem with converting it to 2-nitro-4-chlorophenol is that it needs an autoclave that with operate at 3-5 Bar AND has internal
stirring, my autoclave is rated at 90 bar but doesn't have a facility for stirring. Do you think that I could convert this compound to the appropriate
2-nitro-4-chloroaniline by fusing with urea in a similar method to producing trinitroaniline from from picryl chloride? The aniline would then be
converted into a diazonium compound and hydrolysed to give the appropriate phenol. |
It doesn't seem absurd, but that'll need further researching.
Quote: Originally posted by Boffis  | | In the mean time I have isolated beautiful yellow plates from my crude nitration product, god only knows what it is. I will report back later on
progress and also further investigations into my "4-chlorophenol". |
Yellow colour (to me at least) is highly redolent of nitrated compounds (picric acid, for one, or trinitroaniline you just cited). Given the
propensity of nitrated aromatic compounds to explode, I would approach those crystal warily.
Oh also in attachement an excerpt from Purification of Laboratory Chemicals about chlorophenol.
[Edited on 23-5-2019 by Keras]

|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Thanks for the comments Keras. Waffles ran some reaxys searches for me that give references to the preparation of the nitro and amino sulphonic acid
and it seems that most of the routes discussed above and a few other have been tried. I am checking the references at the moment to see which route
seems most feasible but the route from p-dichlorobenzene looks like a possibility.
If I do go via 4-chlorophenol I will have to prepare it as I mentioned above. I have now investigated my jar of "4-chlorophenol" and it is not a
chlorophenol not even a very impure chlorophenol. the Mp is >100 C, unfortunately my old SM-10 melting point instrument died while I was running
this compound. The compound is fairly soluble in water, the solution is alkaline, it gives a flesh coloured ppt with ferric chloride (no oxidation so
possibly an aliphatic amine), an immediate deep brown flocculent ppt with copper sulphate (no hint of a blue amino type complex) and with sulphuric
acid it generates an intense chlorine like smell though the volume of gas evolved is very small and the smell doesn't persist for long. Further
investigations in progress.
The nitration product is easily separated into 3 compounds. The most abundant is a tiny colourless needles, less abundant but easily purified are the
yellow plates I mentioned earlier and a small amount of thin blades stick to the surface of the water when recrystallized. The yellow plates are not
soluble in sodium carbonate and do not give a deep yellow/orange solution with NaOH either.
This I guess is one of the problems of being an amateur and having to rely on ebay suppliers and the like. You can never be sure what you are getting.
|
|
|
Keras
National Hazard
   
Posts: 768
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Sorry I didn't notice you answered… I'm still flummoxed by the ergonomics of this forum.
I should have a crack at CAS. My uni doesn't subscribe to Reaxys. Oh, it suggested an article called Sulfonation and sulfation in the reactions of
the chloro- and dichlorophenols, 3-fluorophenol and (2,3-,2,4-and 3,4-dichlorophenoxy)acetic acid with concentrated aqueous sulfuric acid and sulfur
trioxide by Peter de Wit and Hans Cerfontain. That's published by Wiley.
Your perplexity reminds me of the old chemists before those sophisticated machines existed. I wonder how those guys during the 19th century were able
to work out the formula of compounds…
I don't buy stuff on eBay. Besides, I don't get anything from China, I source from European dealers only. At least I'm sure to get decent quality
supplies.
Keep me posted!
[Edited on 24-5-2019 by Keras]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Actually the p-chlorophenol I bought was from a guy in Poland from whom I bought quite a bit of material and had very few problems. I have
investigated the material further and have found that it is rather impure but the main constituent is a salt of an a weak organic acid. It is freely
soluble in water with the small amount of brown insoluble material being removed by filtration. The addition of dilute sulphuric or hydrochloric acid
to a hot dilute solution and then cooling deposits a pure white odourless compound that is freely soluble in alcohol but extremely insoluble in water.
This compound cannot be nitrosated and is nitrated only with great difficulty. It burn on foil with a smoky flame and leaves only trace of soot on the
foil. I have a sample drying with the aim of determining water of crystallisation. Once I have an anhydrous compound I will do some quantitative
analysis and determine its acid value.
Back to the original theme: I have checked out all of the references on the Reaxys searches done for me by Waffles and there is some really
interesting chemistry particularly the routes from p-dichlorobenzene. These references lead to an interesting paper in JACS about uses for
p-dichlorobenzene, which was then a waste product, via combinations of nitration and sulphonation. I may come back to this in another thread because I
have so much of it. Several possible routes to the title compound are emerging one from 4-aminophenol-2-sulphonic acid.
This last route is mentioned in passing in one of the German patents as not a cheap (ie commercial) route but it looks interesting if this compound
can be prepared from paracetamol (Tylenol). What do people think about the possibility of sulphonating and de-acetylating paracetamol in one go with
conc sulphuric acid? Do you think the sulphuric acid will oxidize the 4-aminophenol produced by de-acetylation?
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
If sulfuric acid is not concentrated enough then it won't oxidize it.
I don't know, maybe 75-85% acid would do the trick. It would most probably deacetylate and maybe sulfonate in one step as you imagined.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I ran a few more experiments today; 1st I tried out the p-chlorophenol from phenol and sulphuryl chloride then I tried out the sulphonation of
paracetamol.
The chlorination of phenol with sulphuryl chloride worked surprisingly well. The reaction in the cold is slow and I found that I could mix 10g of
phenol crystals with 10ml of sulphuryl chloride (a slight excess) and virtually nothing happened until it was warmed slightly. At about 25-30 C a
fairly vigorous reaction set in and the mixture homogenized into a yellowish liquid and gave of copious amounts of of sulphur dioxide and hydrogen
chloride (needs a good fume hood!!) but the reaction didn't get out of hand. When the evolution of gas ceased, I washed the oily product with water
then dilute sodium hydrogen carbonate solution until it no longer effervesced. I have washed it again with water and I am currently waiting for the
two layer to clear a bit more before I separate and dry the heavy, pungent, slightly orange oil before I distil it. According to the literature
references I have examined some 2- isomer is also formed so the chlorophenol does not solidify at room temperature.
The next experiment was an attempt at the simultaneous hydrolysis and sulphonation of paracetamol to 4-aminophenol sulphonic acid. 10g of
recrystallised paracetamol were mixed with 15ml of sulphuric acid "monohydrate" ie 85% sulphuric acid and warmed until a homogeneous liquid was
achieved, the liquid darkened with time and further heating. The flask was placed on a hotplate at 190-200 C but the internal temperature steadied at
about 160 C +/- a few degrees. After 2 hours the contents of the flask was a black crystalline solid with a distinct smell of acetic acid. It looked
very unpromising but I persevered adding 50ml of water and heating then 7ml of 50% NaOH solution, 1g of decolourising charcoal and finally another
50ml of water before bringing to the boil and filtering hot to obtain a dark brown solution. On cooling nothing precipitated or crystallised so I
added dilute hydrochloric acid until the solution was weakly acid, about pH 4. A thick, flesh coloured, flocculant precipitate formed from the dark
liquor, so I heat and stirred until it was almost boiling and then stared adding more water to try and dissolved the solid (4-aminophenol sulphonic
acid is reported to be sparingly soluble). By the time the solution was at 150ml and boil with much undissolved solid I abandeoned this policy and
left it to cool. A fine but granular crystalline flesh coloured solid has collected at the bottom of the beaker. I will filter it off tomorrow to see
what I have. It is not certain that this is the sulphonic acid as both p-aminophenol and paracetamol are both soluble in alkalis and precipitated by
acids but by all accounts it can be converted via Sandmeyer reaction into the chlorophenol sulphonic acid which is more soluble.
I wonder if it would not be better to mononitrate the paracetamol before attempting to sulphonate it (I suspect that mixed acid will hydrolyse the
acetyl group) in order to make it less sensitive to oxidation? Then carry out Sandmeyer chlorination on the 2-nitro-4-aminophenol sulphonic acid.
|
|
|
| Pages:
1
2 |