| Pages:
1
2 |
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Online
Mood: No Mood
|
|
I got 52 % yield from the H2SO4/HNO3 system, 62 % from P2O5/HNO3 and 77 % from Ac2O/HNO3 (after recrystallisation obviously). However, the yield of
the H2SO4/HNO3 system is artificially low as I accidentally used too much isopropanol in the recrystallisation. Also, I didn't try to optimize the
nitrations; these are first attempts (I didn't have time for anything else).
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
This was a thought of mine, something along the same lines
http://www.sciencemadness.org/talk/viewthread.php?tid=6898#p...
You can see the structural similarity of the molecule on the left
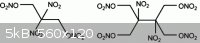
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
glycol solutions density table
There was mention earlier in the thread about distilling ethylene glycol or propylene glycol from anti-freeze.
Attached is a density table which is helpful for determination of the water content of the distilled glycol.
Attachment: glycol solutions density chart.pdf (348kB)
This file has been downloaded 658 times
|
|
|
gnitseretni
Hazard to Others
  
Posts: 280
Registered: 5-1-2007
Location: Medellin
Member Is Offline
Mood: No Mood
|
|
I went to a vet yesterday to get some propylene glycol. The guy said he couldn't legally sell it to me because he didn't have a record of ever having
treated any of my animals.
I thought I'd mention it because earlier in the thread I said you can get it from a vet, but apparently not.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Improved preparation of 2,2-Dinitro-1,3-propanediol
Attachment: 2,2-Dinitro-1,3-propanediol.pdf (137kB)
This file has been downloaded 799 times
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Quote: Originally posted by gnitseretni  | I went to a vet yesterday to get some propylene glycol. The guy said he couldn't legally sell it to me because he didn't have a record of ever having
treated any of my animals.
I thought I'd mention it because earlier in the thread I said you can get it from a vet, but apparently not. |
Find a good vet supply center! I wouldn't go straight to a vet per se'. Your best bet is some place that deals with FFA & 4H members: standard
"stock display" animals. Your using it as a an aid in prevention and treatment of Ketosis (Acetonemia) in dairy cattle. Your dose is about 6oz daily
for an active (or formally active) milking cow.
How does she get Ketosis? Same way as many mammals; too much activity vs. food intake. As a vegetarian with a very differing digestive system; she can
only be milked so much to what she's fed. If she's roaming and them milked too much this can happen. That's why is a big milk farm the girls are in
their stalls most of the time. A milking cow needs rest to produce the best! Running about or getting scared will slow down milk production and
eventually lead to weight loss via Ketosis. That's why you want a contented, relaxed cow.
However there are other uses. In new born mammals it's a soothing washing agent for minor (minor) infections around the umbilical cord. Obviously this
needs to be sterile (USP-grade) but with internal and topical antibiotics; glycol provides an effective method to clean around the "belly-button" in a
new born with minimal discomfort. Frankly, it's used a lot. Some kids use it to put that finishing touch on clean hooves if the glycol is no longer
sterile.
Remember it's going to be sold by the gallon. Don't ask for "anything smaller" because it would simply be inappropriate for it's veterinary usage.
[Edited on 13-1-2011 by quicksilver]
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
I have some experience with PGDN. Somewhat tricky to nitrate (PG easily made rusty fumes), but even with 60 % nitric acid the yields are very good. If
you use the KNO3, (NH4)(NO3) route, the yields are even bigger. It’s volatile, almost as EGDN. The headache from this nitro ester is relative low
compared to NG and EGDN, but still exists, so keep that on mind too. After some misfires and LOD I found that PGDN needs very good kick to do the job,
so a nice compound cap is always a good idea. The speed is not great compared to EGDN or NG, but PGDN is stable and form very powerful (NH4)(NO3)
& flour based dynamites. If you chasing the real McCoy, mix PGDN, NC, acetone and Magnalium (50/50), use strong booster and enjoy the show….
|
|
|
maxidastier
Hazard to Others
  
Posts: 118
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Has someone access to wiley?
I need http://onlinelibrary.wiley.com/doi/10.1002/zaac.200900560/ab... or any methods of synthesis
|
|
|
Polverone
Now celebrating 21 years of madness
|
Thread Split
4-4-2011 at 23:56 |
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Ethylene glycol is also the main ingredient in fog machine liquids.
Supposedly, ethylene glycol dinitrate is up to 10 times less sensitive to friction than nitroglycerin, but I cannot confirm this.
Also want to add that there is another isomer of propylene glycol dinitrate, 1,3-dinitrato-propane. This can be formed from
1,3-propanediol, which is much less vulnerable to destructive oxidation during nitration than common ethylene glycol is, which contains a central
hydroxy group.
1,3-dinitrato-propane has the structure,
O2NO-CH2-CH2-CH2-ONO2
Another obscure route to 1,3-dinitrato-propane is from cyclopropane.
| Quote: |
Cyclopropane derivitives react with a suspension of thallium (III) nitrate in pentane (at room temperature) to give the 1,3-dinitrate esters.
“Formation of nitrate esters by the oxidation of alkenes and cyclopropanes with thallium(III) nitrate in pentane”, Robert J. Ouellette,
Robert J. Bertsch, J. Org. Chem., 1976, 41 (16), pp 2782–2783
http://pubs.acs.org/doi/abs/10.1021/jo00878a035
|
(note that you could just use heptane or kerosene instead of pentane for the solvent)
There is another similar mixed nitro/nitrate ester compound that can also be made from cyclopropane.
| Quote: |
Cyclopropane reacted with dinitrogen pentoxide in methylene chloride at reduced temperature to yield 3-nitro-1-propanol nitrate. (methylene chloride
was just used as the solvent for the reaction)
Golding, Honey, and Miller, US Patent Application 923 024 (1987)
O2NO-CH2-CH2-CH2-NO2
|
To comment on this, these types of compounds have thermal stability problems, meaning they slowly decompose in storage, and the accompanying release
of NO2 could potentially sensitize the substance. Geminal or vicinal nitro groups [meaning two nitro groups on the same or adjacent
carbon atom, respectively] are typically thermally unstable, although there are several exceptions. (Nitro groups vicinal to a nitrato group also tend
to have this problem)
[Edited on 23-1-2012 by AndersHoveland]
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
I have to say Im a bit confused about which PG you folks are refering to.
Rosco,
One of the aforementioned patents you attached for PGDN production (US1371215) did not state what PG was used, although from propylene chlorohydrin
-> PG route mentioned, I guess it should be the common 1,2-PG that was used. Thats correct? (I didnt found on freepatentsonline the application
serial number 178,364 in which this mention is said in the patent).
To what Ive read and what you guys showed here, PGDN/NC gelatines should be stable enough, probably safer than usual NG gelatine.
1,2-PGDN is also used on 'Otto II Fuel' propellant composition along with 2-nitrodiphenylamine and dibutyl sebacate ( http://en.wikipedia.org/wiki/Otto_Fuel_II ).
EDIT:
I found this on Urbanski (vol2 - pag157):
| Quote: | METHYL GLYCOL DINITRATE
Methyl glycol dinitrate (propylene-1,2-glycol dinitrate or 1,2-propanediol dinitrate)
is an oily liquid, boiling at 92°C at 10 mm Hg. Its specific gravity is 1.368 (at 20°C). The liquid does not freeze at a temperature of —20°C. It
is more volatile than the isomeric propylene-l,3-glycol dinitrate.
...
In physical properties and explosive parameters methyl glycol dinitrate resembles
its isomer. The heat of detonation as 1110 kcal/kg (water as vapour). The expansion
produced in the lead block with water tamping is 540 cm3 [4J.
As early as in 1904 the substance was proposed [35] as an additive to lower
the freezing temperature of nitroglycerine, but its practical application on a large
scale was hindered by lack of the raw material, propan-1,2-diol. It is only recently
that the synthesis of glycol from ethylene led to the development of a method for
producing methyl glycol from propylene via chlorohydrin. Even so, propylene-
1,2-glycol is somewhat more expensive than glycols derived from ethylene.
1,2-Propylene glycol was nitrated by Naoum [4] using mixed acid composed of
40% HN03
60% H2SO4
at a temperature of 20°C to produce 187 parts of product from 100 parts of glycol,
i.e. an 86% yield.
A mixed acid containing
47.5% HNO3
45.5% H2S04
7% H2O
was used by Matignon, Moureau and Dode [36] at 10°C. By using 10% excess of
HNO3 they achieved a yield of 91-93%.
|
So probably we all are really talking about 1,2-PG and the yields are quite high.
[Edited on 25-1-2012 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Aqua_Fortis_100%  | I have to say Im a bit confused about which PG you folks are refering to.
Rosco,
One of the aforementioned patents you attached for PGDN production (US1371215) did not state what PG was used, although from propylene chlorohydrin
-> PG route mentioned, I guess it should be the common 1,2-PG that was used. Thats correct? |
Actually the PATR summary indicates it was mixed isomers that was described nitrated by the patent US1371215. But the PATR summary also indicates the
1,2 isomer is less susceptible to oxidation during nitration, so the more commonly available 1,2 isomer which is generally what is called "propylene
glycol" would therefore likely be the safer material of the two isomers for nitration. The label should say what material is present.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Thanks! PATR 2700 (P*398-400) description is very similair (with added info) of Urbanski book's, one quoting another, quite fun.
But, how they know the patent product was mixed isomers? Patent is quite old, 1921. I can just guess that the chlohydrin process cited there in that
time produced the mixed glycols (not separated) used in patent, but that just a guess and I failed to see the serial number.
Concerning oxidation, is cited in PATR (1,3-PGDN) that lower temp than in NG manufacture are required (0 - 10° ... 15-20°C is a no no) in order to
avoid oxidation of central -CH2- group that otherwise is readily attacked.
So, like you said, OTC 1,2-PG is a safer alternative..
It is just interesting/amazing how all of these nitric esters are so compatible with each other, with added/enhanced advantages.. It was further
cited a chief 1,2-PGDN misture containing varying amounts of EGDN and butylene glycol dinitrate, that was commercially avaliable under the trade name
of Nitrobyronel.
EDIT: There is any good info on toxicity of these compounds, especially of PGDN isomers? The best I found is this:
https://docs.google.com/viewer?a=v&q=cache 93Q8HK1rJgJ:www.dtic.mil/dtic/tr/fulltext/u2/766977.pdf+propylene+glycol+dinitrate+filetype:pdf&hl=pt-BR&gl=br&pid=bl&srcid=ADGEESiB
OJkRa4Jwa8_Udsf_cBsyXBbzRYb9WDF8D8uBeSaJLZAT4Mfgs8NLv9xjkpqTAVfL-0JogB5jgh5kVwrvZlMzC6-WO1paRZVjEKq4gDzZT2tukAIwrCN4As06kS936Vf_-98z&sig=AHIEtbRDp
Trvtl4Z04ScT-Qg4LA-UUBJOg 93Q8HK1rJgJ:www.dtic.mil/dtic/tr/fulltext/u2/766977.pdf+propylene+glycol+dinitrate+filetype:pdf&hl=pt-BR&gl=br&pid=bl&srcid=ADGEESiB
OJkRa4Jwa8_Udsf_cBsyXBbzRYb9WDF8D8uBeSaJLZAT4Mfgs8NLv9xjkpqTAVfL-0JogB5jgh5kVwrvZlMzC6-WO1paRZVjEKq4gDzZT2tukAIwrCN4As06kS936Vf_-98z&sig=AHIEtbRDp
Trvtl4Z04ScT-Qg4LA-UUBJOg
"EXPERIMENTAL HUMAN EXPOSURE TO PROPYLENE GLYCOL DINITRATE"
Unfortunatelly I cant get it to load directly to pdf without google visualization.
Quite sad the PGDN comparing to NG seems to pose some added threats to body, especially to eyes..
[Edited on 25-1-2012 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
| Quote: | . . . commercially avaliable under the trade name
of Nitrobyronel. |
And by 'poetic license', I presume? 
P
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
A check of the Wiki article for propylene glycol says that the commercial product
is a racemic (50/50) mix of the 1,2 and 1,3 isomers. It would seem probable to near certainty that it is the cheaper and common 50/50 mix that is
being described by the patents related to the use of the dinitrate of "propylene glycol" as an explosive. There is a different story for the "Otto
Fuel" which specifies the dinitrate of the 1,2 isomer selected specifically for what is reported would be its greater stability, a benefit in the
application for a torpedo propellant.
The Wiki article(s) are not conclusive about the 50/50 ratio although that is what would be expected for a racemic mixture. Anyway the alpha isomer
is the 1,2 and the beta isomer is 1,3 .....that much appears reasonably certain 
CAS 57-55-6 is the designation which comes up on MSDS and product identification and analysis sheets for 1,2 propylene glycol which is the material
designated propylene glycol USP .....the cosmetic, verterinary, and food grade
"propylene glycol" appears not to be a racemic mixture, but appears to be the 1,2 isomer.
Attachment: Dow propylene glycol USP.pdf (114kB)
This file has been downloaded 854 times
[Edited on 25-1-2012 by Rosco Bodine]
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
@Pulverulescent
Yeah, poetic license ending with quite loud gran finale 
@Rosco
Thank you for the info, now Im convinced food grade is most probably 1,2-PG isomer.
Also, I forgot to ask, in the patent they used ammoniacal water to neutralize acidic traces of PGDN, not conventional carbonate/bicarbonate wash,
there is a reason for this other than being chemically better or was made just for economic reasons at that time?
Also, since they used many washs, dried, washed again and dried again, Im wondering what is the solubility of 1,2-PGDN in water at cold or room temp,
it seems probably to be lower of NG and certainly much lower than that of EGDN.
[Edited on 26-1-2012 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The use of dilute ammonia is something I have also favored as a neutralizer but have never seen it explained. The idea I have is that it would serve
to eliminate any unstable traces of organic nitrite ester as byproduct nitrogen or H2O operating similarly as does urea in that regard, as well as
providing acid neutralization. Also the mobility and solubility of the ammonium ion may be better with regards to its ability to penetrate the organic
phase to react with nitrite or acid traces, than would be that property for sodium ion, and the solubility better in the aqueous phase for
transporting the byproduct back into the aqueous phase.....the osmotic pressure or relative solubility there is probably greater and more favorable
for extraction of the ammonium compound byproduct from the organic phase than for sodium compound byproduct. The ammonium ion is volatile also, so
any excess remaining in the organic phase will tend to leave it after neutralization is completed, so it isn't left as an impurity.
[Edited on 26-1-2012 by Rosco Bodine]
|
|
|
freedompyro
holmes1880
Posts: 116
Registered: 16-6-2011
Member Is Offline
Mood: No Mood
|
|
I found the volatility and evaporation issues of EGDN and PGDN makes them far inferior to NG in use. However, EGDN is superior in brisance to NG in
ammonia/nitrate salt dynamites in my experience.
NG is quite a versatile substance as it finds many different uses in rocket fuel, gunpowders, fuses, pyrotechnic coatings, and etc...
In my opinion what you should be looking for is not a safer NG alternative, but a safer way to synthesize NG. It's quite easy to design an apparatus
that either uses compressed air or slow stirring with a plastic blade. The plastic blade must extend into the solution from above to not cause any
areas of friction. A plastic blade set to a speed which will not generate bubbles or microbubbles that turns over the entire solution within 20
seconds is what I use. This in addition to a thermometer probe and addition funnel allows safe control of the nitration. There is no chance of the
common human errors of amateur NG nitration... Eg, thermometer probes banging on the glass, or stirring probes/spoons scraping/hitting the sides.
These errors rarely cause problems but are unacceptable as they create a unnecessary risk factor which has a slight chance of being disastrous.
This badly drawn picture right here should give you an idea of how to design safe equipment for nitration of liquid nitrate esters if you have little
access to glassware. The rubber band runs from the dowel drive to a variable speed motor. You don't have to be an engineer to design a device that
makes nitration of the liquid nitric esters acceptably safe.
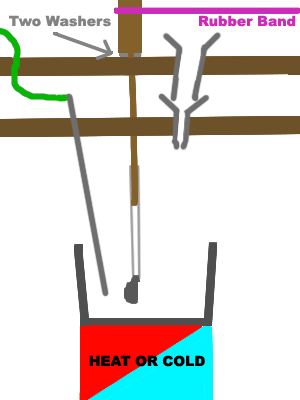
It is even possible to make a blast shield with high grade 1/2"+ thickness plexiglass to work from behind which protects you from injury if working on
a small scale. If you can't get thick high grade plexiglass you could composite multiple sheets together with a clear glue. It must be anchored well!
[Edited on 1-2-2012 by freedompyro]
|
|
|
hames
Harmless

Posts: 24
Registered: 14-2-2012
Location: oz
Member Is Offline
Mood: as was
|
|
Is it possible to nitrate hexylene glycol the same as egdn,pgdn,ng and so forth.also what about ethylene glycol monobutyl ether,sorry in advance if
these are basic questions I just can't find much info regarding nitrating these.
[Edited on 14-2-2012 by hames]
[Edited on 14-2-2012 by hames]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
hexylene glycol should be nitrated, but one of those hydroxy groups are more vulnerable to destructive oxidation. read about nitration of isopropyl
alcohol. the resulting compound should have similar properties to isopropyl nitrate, low sensitivity
http://www.sciencemadness.org/talk/viewthread.php?tid=14492
Only one nitrate group would be added to ethylene glycol monobutyl ether, and the resulting compound would not be an explosive. I am not sure, perhaps
under more rigorous conditions, ethers can cleave apart and be nitrated.
I would think nitration of ethers would potentially be a good strategy because it should absorb some of the water away compared to alcohols. But I
have never read anything about this.
[Edited on 14-2-2012 by AndersHoveland]
|
|
|
hames
Harmless

Posts: 24
Registered: 14-2-2012
Location: oz
Member Is Offline
Mood: as was
|
|
I used to work at a drum recycling yard a few years ago and am back working part time I used to have a 200l drum of 68%nitric and a 200l drum of
sulfuric acid 1840 I collected the bottoms of old drums until they were full so I'm into nitrations,I could collect drums full of 50% hydrogen
peroxide,phosphoric acid,acetic acid glacial,hydrochloric, heaps of methanol,food grade ethanol,toluene,xylene,mek,mibk,ethyl acetate n-butyl
acetate,methoxy propanol,n-butanol ethanolamine triethanolamine dipropylene glycol,hexylene glycol,ethylene glycol,iodine tincture,formalin
plastisizers basically anything that comes in a drum from the cleaning and petro-chemical industry.
[Edited on 14-2-2012 by hames]
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
What about sorbitol hexanitrate? I met sorbitol as a sugar's substitute. According to Jared (I cannot say, that I completely trust him), it is less
sensitive than NG and its preparation is as simple as one of NG. Has anyone tryed to make it?
[Edited on 17-2-2012 by quicksilver]
Women are more perilous sometimes, than any hi explosive.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Quote: Originally posted by caterpillar  | What about sorbitol hexanitrate? I met sorbitol as a sugar's substitute. According to Jared (I cannot say, that I completely trust him), it is less
sensitive than NG and its preparation is as simple as one of NG. Has anyone tryed to make it?
Difficult to maintain it as a solid. Sorbitol nitrates as a layered liquid and doe not crystallize out unless exposed to temperature well below10 C.
Most any warmth will bring it back to a soft near liquid. Difficult to work with and retain neutral pH.
|
|
|
|
| Pages:
1
2 |