| Pages:
1
..
3
4
5
6
7
8 |
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Not yet but it is super easy to cast it. I still have it inton the deep freeze airtide. I will try to cast and detonate few grams like 4g when i got
some time. I also doing some other projects too.
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Should this not be perfect for shape charges due to the high VOD and casting properties? Also for combined effects... any issues with stability if
micron Al is added and mixed in during casting? This would be perfect for detonator bases too.
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
I tried a small cast, 1.5 g, in a plastic tube. Unfortunately the tube deformed. Around 20 minutes at 85-90 degrees did the trick.
What do you guys use for casting into. Is it safe to heat and pour? I just cast it in place. Having read about ETN increased sensitivity when
molten, I was a little cautious.
Aluminum you say? what mesh?

|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
DUN is quite insensitive, you wont have any problem for casting it. Your product thow looks a bit transparent while mine is complitely white solid,
strange. Is it hygroscopic ? Is it solid at room temperature?
[Edited on 2-8-2019 by underground]
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
Quote: Originally posted by underground  | DUN is quite insensitive, you wont have any problem for casting it. Your product thow looks a bit transparent while mine is complitely white solid,
strange. Is it hygroscopic ? Is it solid at room temperature?
[Edited on 2-8-2019 by underground] |
My crystals are also transparent-ish, even before casting. Was yours white even after casting? I'm still wondering if using stronger NA like you are
is an issue. I'm not sure if it is hygroscopic. It looks just like hardened plastic. i did notice a mall area that was still gooey after cooling,
though it seemed to eventually harden.
What do you guys think it will take to detonate this stuff. SADS for example. 60 mg, 100, more...?
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Put some ETN as a booster for detonate. If it goes try then w/ο ETN. After casting it, is it still transparent-ish or not ?
Is there any possibility that there is still some water in there ?
[Edited on 2-8-2019 by underground]
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Whoever detonates this makes history on this board!!
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
I did a cast, around 1.5 g of DAUN, poured into a plastic tube, I put 200 mg of ETN lightly pressed on top. Then around 100 mg of SADS. Will test
tomorrow.
Tested: no detonation. I guess it needs more. Will try with 700 mg.
[Edited on 3-8-2019 by twelti]
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
I now remembered one reason I am wondering about using concentrate nitric acid in the synth. What about leftover acid in the DAUN?? For other EMs we
can easily recrystallize and nuetralize them. How do we do that here? I tested and of course it is acidic. Will it be stable as is? It may be a
pain to reX this stuff to get all the acid out of the crystals. Maybe THAT is why they used dilute NA?
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Your solution should not be acidic. I did not check mine but i really doubt that it was acidic. Did you correctly use your molar ratio ? You may have
added a lot of NA and what you may have now is the Dinitrate salt.
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
Quote: Originally posted by underground  | | Your solution should not be acidic. I did not check mine but i really doubt that it was acidic. Did you correctly use your molar ratio ? You may have
added a lot of NA and what you may have now is the Dinitrate salt. |
I checked twice, with litmus paper. Amount of NA and DAU used was same as in Fischer, though in the last one I used less NA dilution. Could be off
very slightly due to measurement error.
In the last batch, I went to drive off the extra water, and overshot a little. Came to find a white gooey mass. Before, when I melted crystalline
DAUN to cast it, it was clear. This stuff was opaque white. When it cooled off it was still a very slightly gooey in parts. I'm thinking there was
still water in it. I added some water to the gooey white mass and it readily dissolved. Then I added Methanol and it came out of solution. Looked
like curdled milk. So I guess this is how it could be recrystalized if that were necessary.
EDIT: looks like the DAUN does not really dissolve in water, since upon standing the water separates. It's a little confusing.
[Edited on 4-8-2019 by twelti]
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
From pdf they claim that the salt got high density due to hydrogen bonds between the molecules. I believe the same is happening with water, and maybe
that is why it is hard to crystalise out, even thow the solubility is not that great.
[Edited on 4-8-2019 by underground]
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Surly Rosco has some input on this thread...?
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
Wherefor art Thou, Sir Roscoe?
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
I have tried a couple of times, to detonate some of this. Twice w cast and once with pressed powder. Seems to want a healthy kick to get it
going...?
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by twelti  | | I have tried a couple of times, to detonate some of this. Twice w cast and once with pressed powder. Seems to want a healthy kick to get it
going...? |
So did you actually detonate it ?
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
The world is waiting....
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
I'm not 100% sure. I have been using the steel conduit box cover plates that you buy at the HW store. compared to what 700 mg of PETN does to it,
the damage from around 2.5 g of DAUN didn't look to be enough. I think I need a larger diameter charge.
Here is my latest batch. 6.5 ml 70% NA, diluted to 40 ml with H2O, and added 9 g of DAU. I gently boiled it down to 20 ml, then vacuum down to 15
ml. Then I added EtOH, up to 80 ml. It seems that at least once when I quickly poured in EToH, I got a lot of crystals fairly quick. This time I
added it gently and din't stir. Nice layer of oily DAUN at the bottom. Even after I eventually it did stir it, it settled back down.
What does the alcohol DO? Shuld we be using EtOH or MeOH or even something else?
I'm still wondering about the concentration of the NA. Does the actual concentration matter? Or is it purely the amount of NA, irregardless of how
much water there is? Could one go the other way and use WFNA then, and have NO water to remove? Probably a bad idea but....

|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I think diaminouronium nitrate really needs a water molecule to crystallize, ethanol and methanol either pull of water molecules or dilute the water
available (while the crystals not being soluble in the alcohol), making the crystallization possible.
I'm interested in a reaction, theory all over, where methanol/ethanol is used as a solvent and the needed nitric acid is added with just a 1:1 molar
amount of water. Keep this reaction below zero during the first try!
Edit: dissolve the base in ethanol/methanol --> cool in a freezer --> put on ice --> add 50:50 molar water/HNO3.... see what happens. My
guess: instant crystallization of the mono-hydrate.
| Quote: | | I'm not 100% sure. I have been using the steel conduit box cover plates that you buy at the HW store. compared to what 700 mg of PETN does to it, the
damage from around 2.5 g of DAUN didn't look to be enough. I think I need a larger diameter charge. |
How do you detonate? What primary do you use?
I think it dissolves one (or more) of the products and forms an "organic layer". I see the same when reacting benzaldehyde/acetone/NaOH/water. I get
a separation that is not linear with one of the reactants.
[Edited on 9-8-2019 by Tsjerk]
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | I think diaminouronium nitrate really needs a water molecule to crystallize, ethanol and methanol either pull of water molecules or dilute the water
available (while the crystals not being soluble in the alcohol), making the crystallization possible.
I'm interested in a reaction, theory all over, where methanol/ethanol is used as a solvent and the needed nitric acid is added with just a 1:1 molar
amount of water. Keep this reaction below zero during the first try!
Edit: dissolve the base in ethanol/methanol --> cool in a freezer --> put on ice --> add 50:50 molar water/HNO3.... see what happens. My
guess: instant crystallization of the mono-hydrate.
| Quote: | | I'm not 100% sure. I have been using the steel conduit box cover plates that you buy at the HW store. compared to what 700 mg of PETN does to it, the
damage from around 2.5 g of DAUN didn't look to be enough. I think I need a larger diameter charge. |
How do you detonate? What primary do you use?
I think it dissolves one (or more) of the products and forms an "organic layer". I see the same when reacting benzaldehyde/acetone/NaOH/water. I get
a separation that is not linear with one of the reactants.
[Edited on 9-8-2019 by Tsjerk] |
Thanks for your comments. I will try your suggested approach and report back. As for primary, i was using SADS+KCLO3, then 600 mg of PETN. I think
maybe it just needs a bigger kick and/or larger diameter tube.
Actually, when you are you talking about the monohydrate, are you talking about the dintrate monohydrate, i.e. [3] from Fischer? that is the less
interesting version as I understand it. The nitrate is the one we want.
[Edited on 9-8-2019 by twelti]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
No, I'm talking about they mono-nitrate monohydrate, I didn't dubblecheck but from what I remember both the nitrates crystallize as the mono-hydrate.
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
BTW I found this patent which is related
US2970899A
This sort of describes what happened to my when I boiled the product solution down too far:
in attempting to dehydrate the carbohydrazide mononitrate by lheating C.), it was found that water was driven off. At the cessation of this water
evolution, heating was discontinued. The distillate was opaque milky white and very viscous. Upon standing, it scparated into two phases into a clear,
colorless, viscous liquid and the white solid. It was found that the solid constituted about 18% of the total weight of the distillate. Repeated
attempts at analyzing and classifying this substance were unsuccessful. It was assumed that the solid material was either a decomposition product or a
product of molecular rearrangement or both.
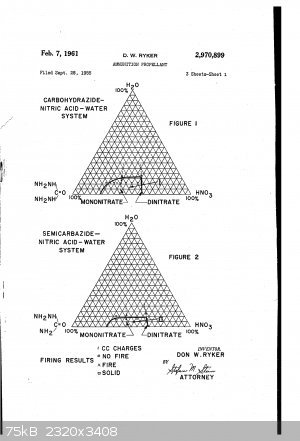
[Edited on 10-8-2019 by twelti]
|
|
|
MineMan
National Hazard
   
Posts: 998
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Does this mean one does need 40 percent or less HNO3 for the mononitrate?
I see. So we can’t boil it? Guess that’s why you’re did not det
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Hmm interesting. That is why my product was a solid white. It possibly must be a molecular rearrangement or the unhydrate form since i did not heated
it that much to decompose the whole product and the whole product was that white solid. In addition the decomposition temp of mononitrate is 242 C and
i really doubt that i reached that temp.
[Edited on 10-8-2019 by underground]
|
|
|
twelti
Hazard to Others
  
Posts: 217
Registered: 20-2-2019
Member Is Offline
|
|
I'm not sure, but I think now that white stuff might be an EM too. I put around a g in a small tube w 700 mg of PETN and I think it did detonate.
I'm not 100% sure. I need to try a larger amount.
The whole thing is driving me nuts since I have done this maybe 7 times and on one attempt I got a pretty good and quick yield of crystals, but not
positive what I did different. I WILL find out though. If anyone wants to try, and doesn't have the carbohydrazide, I can send some. I have plenty!
|
|
|
| Pages:
1
..
3
4
5
6
7
8 |