| Pages:
1
2 |
reactofurnace
Hazard to Self
 
Posts: 76
Registered: 17-7-2015
Member Is Offline
Mood: Volatile
|
|
Benzaldehyde By Reduction Of Benzoic Acid
Hey. I was wondering if it is feasible to reduce benzoic acid to benzaldehyde or benzyl alcohol. I do not have sodium hydride, sodium borohydride or
lithium aluminium hydride. I was hoping to do it with other more accessible reducing agents. Any suggestions?
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Hmmm. The reduction will not be easy. And in case you're wondering, the Cannizzaro reaction isn't reversible, so that won't work. Besides the
hydrides, I don't know of any reducing agents powerful enough (and frankly, using hydrides would be a waste).
If you have toluene you could try doing an oxidation. There's a stickied thread on this forum.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
This may be more interesting than useful but...
https://www.sciencedirect.com/science/article/abs/pii/S09268...
This might be more accessible to the home chemist.
http://jes.ecsdl.org/content/75/1/411.abstract
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
I was wondering today why none has tried making benzaldehyde by the Stephen aldehyde reduction of benzonitrile which at first glance seems easy to
make from benzoic acid and an ammonia source such as ammonium or urea. Some quick searching lead me to the conclusion it is in fact not that simple to
form the nitrile but perhaps it is a viable path.
EDIT: Maybe phenyl group is too electron withdrawing for Stephen
[Edited on 3-7-2019 by Σldritch]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
You could make the ester, and react it with a Grignard to get a ketone or alcohol. Not easy, though.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
It´s possible to reduce benzoic acid to the aldehyde with hydrogen in a tube funace.
User Organikum posted sth about it on the hive i think.
[Edited on 3-7-2019 by DrScrabs]
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Distill benzyl alcohol from paint stripper (it's in everything now that DCM has been demonized), then oxidize that with Manganese dioxide from
alkaline batteries.
You can thank me as your personal jesus later.
/CJ
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
reactofurnace
Hazard to Self
 
Posts: 76
Registered: 17-7-2015
Member Is Offline
Mood: Volatile
|
|
This is indeed really interesting. I like the electrolytic reduction idea. I don't want to deal with lead or cadmium electrodes and possible oxides
that may form tho.
If I get more information I could try it with another electrode material. I don't mind lower yields since benzoic acid is really easy to make.
Thanks.
|
|
|
reactofurnace
Hazard to Self
 
Posts: 76
Registered: 17-7-2015
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by Corrosive Joeseph  | Distill benzyl alcohol from paint stripper (it's in everything now that DCM has been demonized), then oxidize that with Manganese dioxide from
alkaline batteries.
You can thank me as your personal jesus later.
/CJ |
Looool. Yes that's a great option
|
|
|
RogueRose
International Hazard
    
Posts: 1585
Registered: 16-6-2014
Member Is Offline
|
|
Benzyl aclohol is very easy to get and is sold w/o question at most online retailers who supply perfume scents. They sell from 100ml to 5gal drums,
shipped with no questions last time I checked. IDK where you live but I think it's even sold on ebay. Wouldn't this be a much easier and cost
effective method than using hydrides and acid?
https://www.medical-and-lab-supplies.com/benzyl-alcohol-usp....
1 gal - $60 - free shipping
https://www.amazon.com/Gallon-Benzyl-Alcohol-Sterile-Plastic...
Here's a link for a few different methods of doing the conversion. There is also a description/process of how to do it with toluene which might be
easier or less expensive at least.
[Edited on 7-4-2019 by RogueRose]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
You could have a look at the oxidation of benzyl alcohol with dilute nitric acid, I haven't tried it but as discussed somewhere here it seems quite
doable.
Edit: There are some papers referenced in this discussion using 10% nitric acid.
I wouldn't start with benzoic acid unless you really have to, because the most efficient way would probably be through the alcohol anyway.
[Edited on 4-7-2019 by Tsjerk]
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
I think that the oxidation of benzyl alcohol with dilute nitric acid and just a touch of a nitrite salt, is the ticket.
There is the worrisome byproduct of benzyl nitrite which might be problematic to pull from the main product, may be forced to do the adduct to get it
removed.
I believe that the boiling points are pretty close
I should have already done this and worked through the workup but life sometimes makes for busy times.
[Edited on 7/4/2019 by morganbw]
[Edited on 7/4/2019 by morganbw]
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i have just had a small scale success of reducing benzoic acid with ascorbic acid, along with a few drops of HCl
i tried initially with Zn+HCl but it gave me nothing
with the benzoic + ascorbic acid there was a slight hint of benzene present, but it was only done in roughly 5 milliliters, as carelessly as just
dumping it in oven for an hour at 100*C
i have done the benzyl alcohol to benzaldehyde using (5%) nitric acid and some catalytic amount of sodium nitrite
and ive kept the resulting.. 20mL in fridge for maybe 5 years now, the upper layer is a strong yellow and smells like almonds while the bottom layer
is likely benzyl nitrite, the reaction is forming benzyl nitrite which then decomposes into benzaldehyde, so the benzyl nitrite shouldnt be an issue-
and it seems to store quite well, cranking it open every now and then doesnt yield much if any decomposition gasses so its quite stable to store in
fridge.
|
|
|
Texium
|
Thread Moved
17-2-2022 at 11:28 |
j_sum1
Administrator
       
Posts: 6220
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I can state that starting with toluene is not easy.
Whatever you do, if you oxidise from toluene or the alcohol, ypu need to select your oxidant carefully so you don't overshoot and get the acid. Cr(VI)
is classic but there are other options.
I suspect a reduction route will be a lot harder.
|
|
|
Keras
National Hazard
   
Posts: 769
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
You might get a chance to oxidise benzyl alcohol to benzaldehyde in a dry solvent. Aldehydes react with water to form hydrates (diols)
-COH + H₂O ⇌ -C(OH)₂ + H⁺. This reaction has not a very high K, so the amount of diol formed is minimal, but the oxidising agent will attack
the diol to form the carboxylic acid: -C(OH)OH → -C(O)OH, and since this reaction is not reversible, it drives the former ahead, progressively
oxidising all the aldehyde into the carboxylic acid.
You can try PCC in DCM. I even saw a paper about oxidising benzyl alcohol to benzaldehyde using bleach and a phase transfer catalyst (TBAB in this
case).
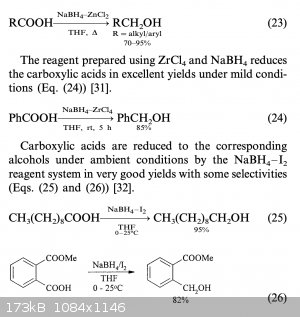
Reducing a carboxylic acid can be done with lithium aluminium hydride, that brings you back to the alcohol. Granted LiAlH₄ is not easy to source.
You can use the easier to source sodium borohydride with zinc chloride, apparently. See attached document.
I wonder if sodium dithionite would work. It is known to reduce aldehydes to alcohols above 80°C, so that might work.
In any case, all these reactions would bring you back all the way to the alcohol. I don’t think it’s possible to stop at the aldehyde level, given
the reactivity thereof.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
so- water makes this reaction more difficult? im having some success with ascorbic acid and benzoic acid, it seems that the ascorbic acid decomposes
above 80*C so i keep it at 75*C
its not a very high yield if any yield at all yet, but it does indeed reduce it to benzaldehyde
so im at the proof of concept stage, now if we could find a solvent that doesnt interact with the reduction reaction or the benzoic acid that would be
next step
maybe a catalyst of some sort would help this.
"Ascorbic acid (vitamin C) is an unusual antioxidant in that it donates a single reducing equivalent, and the radical it forms, monodehydroascorbate,
reacts preferentially with radicals instead of with non-radical compounds."
ascorbic acid is soluble in EtOH at 20g/L while benzoic is 25g/100mL, that should work and then the boiling point of ethanol would limit the
temperature to be just about below decomposition temperature of ascorbic acid
ascorbic acid may at a slow rate react with the ethanol, as may the benzoic acid forming "ethyl ascorbic acid" which is apparently a reducing agent of
some sort, and ethyl benzoate, but with only weak acids present this should be slow
im about to find out.
|
|
|
Keras
National Hazard
   
Posts: 769
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
This document can be useful, too.
Attachment: The Syntheses of Aromatic Aldehydes.pdf (1.8MB)
This file has been downloaded 312 times
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i just got a hint "dry distilling calcium benzoate and formate together yeilds benzaldehyde"
i dont have the means to test this one out just yet, but the guy that messaged it to me might soon
its likely in temperature range of 200-300*C and benzaldehyde boils at about 200, and also oxidizes quite fast. maybe adding in some formic acid could
help to keep the benzaldehyde well reduced, short distillation tube? i personally dont have equipment for vacuum distillation which is normally
advised for distilling benzaldehyde to prevent oxidation
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Turning benzyl alcohol into benzaldehyde seems to be a common video on YouTube.
I'd suggest looking for these vids to get an idea of which route to take.
As suggested above now that dcm is out of otc paint stripper benzyl alcohol is in and
BzOH is on eBay making this available to everyone. Toluene and potassium permanganate is another option if one can just accept the low yeilds and keep
it under 40'c.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Obtaining benzyl alcohol and oxidizing is definitely your best bet, or buying benzaldehyde directly from a chemical supplier. I wouldn't recommend any
method suggested that involves dry distillation of benzoates. My three favorite oxidizers for benzyl alcohol are, in order: nitric acid, persulfates,
potassium chlorochromate. I recommend a large batch size because it improves the ease of purification via the formation of a bisulfite adduct. It can
be stored indefinitely as the bisulfite adduct and converted back to benzaldehyde on demand if you only plan to use a little benzaldehyde your first
go. If you choose to distill your benzaldehyde for extra purity after the bisulfite adduct has been broken, I recommend a kind of steam distillation
where neutral pH water is boiled with a layer of benzaldehyde on top, carrying over the benzaldehyde as droplets at a low temperature. Vacuum is
obviously the first choice but this isn't easy for everyone.
Chem Player's channel on bitchute has some good videos to watch on these topics, and they themselves are members of the forum here, so you're far from
alone on this topic.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
these inputs dont pertain to the question originally asked, or rather the challenge
its not difficult to make benzaldehyde if you have benzyl alcohol
everything is readily available if you wanna put yourself on a watched list
and, everything is legal until some authoritative figure starts asking you questions
there has been cases where people got a visit for simply ordering copper sulfate- i suppose its a learning experience from some, because it appears
some people are completely oblivious to the fact that many chemicals are controlled - and thats why, people ask questions such as, how can we turn
chemicals that are not watched or controlled by any means- into chemicals that are
im not even the man asking this question originally but its frustrating, and actually- way too common to see this kinda response on a forum, it should
go against rules to trash the original question and suggest a route using a less available chemical thats for obvious reasons being avoided.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Benzyl alcohol and bleach are not suspicious, and I think the reduction of benzoic acid is not really viable. At least not for those who can't just
buy benzaldehyde. When you can't get benzaldehyde it makes a lot more sense to discuss going from the alcohol instead of the acid.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by Antiswat  | i just got a hint "dry distilling calcium benzoate and formate together yeilds benzaldehyde"
i dont have the means to test this one out just yet, but the guy that messaged it to me might soon
its likely in temperature range of 200-300*C and benzaldehyde boils at about 200, and also oxidizes quite fast. maybe adding in some formic acid could
help to keep the benzaldehyde well reduced, short distillation tube? i personally dont have equipment for vacuum distillation which is normally
advised for distilling benzaldehyde to prevent oxidation
|
Apparently this doesn't work in real life. Possibly might work under argon or vacuum atmosphere otherwise benzoic acid is the only thing that distills
over
|
|
|
Keras
National Hazard
   
Posts: 769
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
You all know that famous reaction (now also featured in one's of NileRed shorts), called in French “lampe sans flamme”, in which a wire or ribbon
of red-hot copper is suspended over a beaker full of ethanol vapour, producing ethanal and remaining hot because of the exothermicity of the
oxidation.
I tested the same reaction with isopropanol, btw, and it works even better, it seems. Now I wonder what is produced: acetone or ketene?
Just to come back to our thread: would the same experience work if ethanol was replaced by benzyl alcohol, leading to benzaldehyde (with a bit of luck
I’ll be able to test this later this week).
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
Quote: Originally posted by Keras  | You all know that famous reaction (now also featured in one's of NileRed shorts), called in French “lampe sans flamme”, in which a wire or ribbon
of red-hot copper is suspended over a beaker full of ethanol vapour, producing ethanal and remaining hot because of the exothermicity of the
oxidation.
I tested the same reaction with isopropanol, btw, and it works even better, it seems. Now I wonder what is produced: acetone or ketene?
Just to come back to our thread: would the same experience work if ethanol was replaced by benzyl alcohol, leading to benzaldehyde (with a bit of luck
I’ll be able to test this later this week). |
From the videos I've watched, this reaction appears to work the best with acetone, with ethanol being a poor second. If you used isopropanol, I'm
guessing you got acetone first of all, then that was probably oxidized second as well, which would explain why it worked better.
Not sure what reaction might occur with benzyl alcohol, but one thing to note (as you probably have already) is that benzyl alcohol boils at over
200C. So you will definitely need to heat it to get an appreciable amount of vapor for the reaction.
|
|
|
| Pages:
1
2 |