mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
Dimethylethanolamine ??????
Hello,
I'm wondering because Picric-A said that reaction 2-chloroethanol with excess dimethylamine for sure gives 2-Dimethylaminoethanol , but i search more
"healthy" reaction and i found this (in Survey of industrial chemistry by Philip J. Chenier) :
acetaldehyde + dimethylamine ----------> Dimethylethanolamine ?
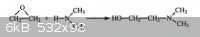
Is this really can works ??????????????????? Please help, for any help thx v. much. 
|
|
|
TheOrbit
Hazard to Self
 
Posts: 59
Registered: 4-4-2009
Location: deepth point of a black hole
Member Is Offline
Mood: manic bipolar science dis/order
|
|
you mean ethylene oxide " not aldehyde"
http://www.patentstorm.us/patents/5663444/description.html
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
yes yes ethylene oxide , thank you
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Works. Ethylene oxide is very toxic gas. It is also a very potent carcinogen.
Use ethylene chlorohydrin instead. Just as toxic, put not very volatile, so it is easier to store and work with.
|
|
|
mario840
Hazard to Others
  
Posts: 229
Registered: 20-1-2010
Member Is Offline
Mood: No Mood
|
|
So just 1 mole 2-chloroethanol and 1 mole dimethylamine and reflux for how long ? you know something more about this reaction ? i will be greatfully
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
No, it would be 2 mol dimethylamine to 1 mol ethylene chlorohydrin.
ClCH2CH2OH + dimethylamine --> ethylene oxide + dimethylammonium chloride
Ethylene oxide + dimethylamine --> dimethylethanolamine
I don't have any experience with this specific case, but I have some with ring opening of aziridines. If I were to do it, I would add the
chlorohydrin drop-wise to the commercial 40% aqueous dimethylamine solution cooled on an ice bath. I would use a healthy excess of the dimethylamine
(3x or more). After addition allow the stuff to stir for an hour or so. Assuming the Dimethylaminoethanol does not form an azeotrope with water
(which it may) purify it via distillation.
|
|
|