| Pages:
1
..
15
16
17
18
19 |
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
So, we've got a source of alpha-terpineol. What's our next step, blogfast? Or, at least, what progress have you/we achieved?
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
After reduction of the double bond a possible substitute for tBuOH is obtained, which is sterically more demanding and has a higher boiling point. The
double bond, if not reduced, may lead to decomposition/polymerization. Maybe it's worth a trial using alpha-terpineol directly and look what happens.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Velzee  | | So, we've got a source of alpha-terpineol. What's our next step, blogfast? Or, at least, what progress have you/we achieved? |
Most of the work done so far (on pinenes) was done by aga (under my direction) and is reported higher up in the thread. Poor health and other issues
mean I won't be touching a test tube until about 2017.
Guess I'm looking for someone to carry the torch? Interested? 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Alice  | | After reduction of the double bond a possible substitute for tBuOH is obtained, which is sterically more demanding and has a higher boiling point. The
double bond, if not reduced, may lead to decomposition/polymerization. Maybe it's worth a trial using alpha-terpineol directly and look what happens.
|
All of that is discussed higher up, including using neat alpha-pinene and hydrogenation of the relevant carbinol. Worth taking a gander, IMO.
Certainly our 'blueprints' were sound but execution met with numerous problems.   
Personally I'm still very interested in developing a much better catalyst for the reduction of KOH with Mg in paraffinic media. And for NaOH/Mg, of
course!
Any assistance remains appreciated.
[Edited on 27-8-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The pinene related stuff starts getting interesting from about here:
http://www.sciencemadness.org/talk/viewthread.php?tid=15171&...
[Edited on 27-8-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Today's 'progress' was yet another failure to create chloral as a precursor to trichloroacetic acid, which supposedly catalyses the pinene reaction.
20 hours and 260 litres of chlorine later ...



The resulting 2-phase liquid was heated to 80C on a water bath (to drive off any remaining ethanol & dissolved chlorine), then had an equal
volume of conc sulphuric acid added.
The liquid remained biphasic, so they were separated, then the upper layer was weighed and had a stoichiometric amount of water added.
This just made it wet.
No crystals, nothing.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@aga:
Welcome back!
Let's recap before we carry on.
Soon!
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
I had a look at a paper mentioned previously:
"Synthesis of terpineol from α-pinene by homogeneous acid catalysis"
http://www.sciencedirect.com/science/article/pii/S0920586105...
In the abstract chloroacetic acid and HCl are mentionend, but in the paper acetic acid, and oxalic acid as well.
Reaction conditions for oxalic acid:
4 h, 70 °C, 0.6 mol water, 0.25 mol alpha-pinene, 0.43 mol oxalic acid:
Conversion: 39.8 %
Selectivity: 60.8 %
=> 24% yield
My intuition says the numbers have an error, because there is a strong trend of increasing conversion for 0.03, 0.11, 0.22, and for 0.43 mol oxalic
acid conversion is reported lower than for 0.22 which seems unlikely.
[Edited on 29-8-2016 by Alice]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Getting the reaction mixture anywhere near homogenous was really hard.
At one point i even added a few drops of detergent to try to break the oil up.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Can you summarise that last run?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I'll go and dig thru the notes.
Will take a little time.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK. Trawled thru a pile of random notebooks and cannot find as much info as there is in this thread about what i did.
Reading it all (again) i think i simply ballsed up the whole thing due to inexperience.
Most likely errors appear to be :-
Lack of detailed notes (!)
Poor Mixing technique.
Inadequate Temperature control.
Impure reagents.
Poor Lab hygiene.
Careless Handling.
By sheer luck there were some (very few) crystals after the first attempt.
I suggest we go back to the start with a slightly more experienced operator and try that procedure again.
If Alice (and anyone else interested) would also please try it, then we can compare notes.
[Edited on 29-8-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@aga:
Do we have a plan?
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
Aga, can you remember how you achieved crystallization?
Considering the reported tiny amount of water being very near the molar range of oxalic acid, this might have a considerable impact on the reaction.
After having had a closer look the example I was wondering about, lower conversion but more oxalic acid than the following shows something else:
4 h, 70 °C, 0.6 mol water, 0.25 mol alpha-pinene, 0.22 mol oxalic acid:
Conversion: 53.4 %
Selectivity: 64 %
What I've ignored is the fact that the difference isn't just the oxalic acid amount but the altered oxalic acid : water ratio. 1 : 1.4 vs. 1 : 2.7.
Additionally the authors mention, the reaction to proceed mainly at the pinene/water interface due to bad solubility. So, why not enlarging the
interface by using more of the more favorable ratio, i.e. 0.43 mol oxalic acid with 1.16 mol H2O? It is strange, that the reaction stops at 53.4%
conversion isn't it? More interesting, the product doesn't really decompose after a prolonged heating time.
Anyhow, the reaction goes probably better the finer oxalic acid is ground.
My suggestion is:
2 h, 70 °C, 0.3 mol water, 0.25 mol alpha-pinene, 0.43 mol oxalic acid dihydrate.
How is the plan for reduction of the double bond?
[Edited on 30-8-2016 by Alice]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The 'product' was just left to stand overnight and there were a few crystals the next day.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Assuming the unsaturated carbinol (2,2-dimethyl something unsaturated-2-ol), pictured in the pic of this post of the thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=15171&...
... can be obtained in sufficient quantity then I believe that hydrogenation with Pd/C catalyst and ammonium formate must be possible and quite easy
to perform.
The right hand side carbinol is the one to be hydrogenated:
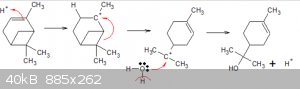
[Edited on 30-8-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Not got any Pd/C catalyst, but then, not got past just having a bottle of turps at the moment.
Running a Cl2 generator for many hours to get nothing useful was a big waste of time (not to mention the cleanup time !).
No oxalic acid here, so are there any other possible catalysts that i might have ?
If not, maybe better to just attempt the synth on page 9 again (starting with the fractional distillation on page 8, of course ).
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Astonishing !
In a book published around 140 years ago, oxalic acid was produced by heating pine sawdust with KOH/NaOH, citing an earlier ref to 'Thorn' (no date
given).
1:2 ratio pine:KOH, heat to 250 C with stirring, lixiviate etc.
Page 199, Manual of chemical technology, Rudolf von Wagner, Johannes Rudolf, 1822-1880.
https://archive.org/details/manualofchemical00wagnuoft
Found a few methods on utoob using sugar and HNO3, but the sheer volume of NOx produced makes them a LOT less attractive.
Pine and KOH/NaOH i have, so time for some chainsaw action.
(maybe the bench drill would be better so no lube oil gets in the sawdust)
OK, so may as well give Alice's method a whirl.
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
Oxalic acid is available in hardware stores for rust removal and as optical brightener for wood.
Pd/C is unavailable for me too as well as any kind of platinum catalysts, raney nickel. What seems available is urushiba nickel, but I'm afraid it's
way too weak.
Seems like a deadlock, even if it would be performed by someone it remains difficult to access.
I think I'll go back to camphor with some oranozinc reagent and hope for less reduction product. 
@aga, I like these old methods!
[Edited on 30-8-2016 by Alice]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Alice  |
Pd/C is unavailable for me too as well as any kind of platinum catalysts, raney nickel. What seems available is urushiba nickel, but I'm afraid it's
way too weak.
Seems like a deadlock, even if it would be performed by someone it remains difficult to access.
|
Nah. Get it from eBay, Amazon or prepare it yourself. Not hard at all.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Erm, given that is an Amateur attempt, and that we got some (very few) crystals once, i'd suggest we do that procedure again, more carefully.
What 'carefully' means is also unknown, so any suggestions welcome.
Presumably Alice will also be conducting the experiment, which will be very useful in determining the best way to go.
It's still 37 C here in the day, which is why i suspect temperature control in all OC synths is one of the Key factors to 'getting it right'.
(cite: multiple chloral synths in summer failing and one when it was cooler getting at least a solid)
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Why not get limonene instead of terpineol and then add two moles of H2O to it to get a diol
the more OH on the molecule,the better 
how to extract limonene from orange peels - https://www.youtube.com/watch?v=o4CBXkfVHDc
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by CuReUS  | Why not get limonene instead of terpineol and then add two moles of H2O to it to get a diol
the more OH on the molecule,the better  |
Limonene extraction looks easy enough.
Any references for 'adding mols of water to limonene to get a diol' ?
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
@CuReUS
If double hydration on limonene would work, it would work on terpineol as well, right?
I'm not convinced about "the more OH the better" because the goal is good solubility of the corresponding alkoxide in alkanes. The only way I can
imagine a diol might work is one that has chelating properties, like 1,4-diethyl-1,4-dihydroxycyclohexane which might form a soluble dimer or
something similar as an alkoxide.
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Nah. Get it from eBay, Amazon or prepare it yourself. Not hard at all. |
@blogfast, I don't have what could be called a lab, a fume hood, inert atmosphere, and so on. Unavailable means, I had to buy all the stuff and
equipement too including a house with a cellar, a garage, a garden or whatever and that's simply too much. 
[Edited on 31-8-2016 by Alice]
|
|
|
| Pages:
1
..
15
16
17
18
19 |