| Pages:
1
..
12
13
14
15
16
..
19 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | In this JPL paper
https://www.researchgate.net/publication/231665911_Heterogen...
they say :-
"Acetone was found to be physically absorbed by sulfuric acid without undergoing irreversible reaction below acid concentrations of 87 wt. %"
Above 87 wt % they saw 'condensation products' such as mesityl oxide.
This suggests that the 'simple' mix of acetone and our sulphuric acid catalyst is far from simple.
|
Firstly, be careful to quote papers that are very far removed from our actual experimental conditions.
At very high acid concentrations, even acetone become protonatable, but these concentrations are far removed from ours.
No, the main reason for our poor yields is almost certainly further hydration of the terpineol on the second double bond. It's mentioned in
most papers related to terpineol synthesis and it happens with TCA catalysis too!
[Edited on 10-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Currently a bit lost in contemplating what even H2SO4 and H2O do at the electron/quantum level.
That paper was cited simply to point out that the reaction mechanisms are not Simple, with the reaction of the catalyst and the solvent as an example.
Yes, the 2013 paper categorically shows reduced yield after 30 mins with TCA.
What second double bond ?
Can only see one double bond (resonant) in the pinene or the terpineol.
Can see two in the SO4<sup>2-</sup> anion, although likely dislocated and resonant as well.
[Edited on 10-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
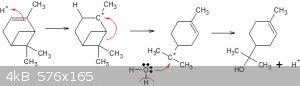
Sorry, by 'second' I meant that the left side double bond in the pinene disappears, then reappears to the right of it when the carbocation moves.
That double bond in the terpineol is also hydratable but less easily than the 'first'. Neither of these bonds is resonant BTW: neither are
conjugated!
| Quote: | | Currently a bit lost in contemplating what even H2SO4 and H2O do at the electron/quantum level. |
As long as there's water, the acids completely deprotonate. The H3O<sup>+</sup> is the REAL catalyst.
[Edited on 10-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I do not know enough to propose an alternative reaction mechanism.
Logic dictates that it cannot be as simple as your proposed reaction mechanism suggests, simply because the Temperature, Time, Catalyst and the
Solvent all have a strong influence on the concentrations of the product species, which implies that they are all Involved in the reaction.
Specifically, external or additive intermediate species form (not just carbocations) which produce alpha-terpineol via a route that involves water,
the acid catalyst and the solvent.
TCA as catalyst producing more alpha-terpineol in less time than sulphuric acid is surely evidence of that : they both disassociate, giving protons,
yet TCA works better.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
TCA as catalyst producing more alpha-terpineol in less time than sulphuric acid is surely evidence of that : they both disassociate, giving protons,
yet TCA works better. |
We only have the author's assertion for that: no comparison with other catalysts in perfectly comparable conditions has been presented. Nor have I
found one elsewhere.
It really is a bit like saying: 'Esso petrol works best' w/o offering a back-to-back comparison with 'Shell', 'Fina' etc etc.
There's no meta-analysis making that claim either. Scientifically the burden of PROOF for such claims MUST be high.
That paper on TCA is actually quite broad in its claims but very poor on detail.
'The Devil is in the detail'
| Quote: | | Logic dictates that it cannot be as simple as your proposed reaction mechanism suggests, simply because the Temperature, Time, Catalyst and the
Solvent all have a strong influence on the concentrations of the product species, which implies that they are all Involved in the reaction.
|
True but you're using quite a broad definition of 'reaction mechanism' here: a full description of catalysis, activation energy, influence of
concentrations etc can of course not be included in a simple qualitative description of changed bonds during the reaction path.
[Edited on 10-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by blogfast25  | | We only have the author's assertion for that: no comparison with other catalysts in perfectly comparable conditions has been presented. Nor have I
found one elsewhere. |
Reference [9] in the 2013 paper is a 2005 $40 article :-
http://www.sciencedirect.com/science/article/pii/S0920586105...
The precis mentions chloroacetic acid and HCl, and might not mention any more than that.
Feck 'em. Forge on and actually make some.
[Edited on 11-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The abstract of that paper:
| Quote: | | Chloroacetic acid was used as catalyst for the hydration of α-pinene using water as hydroxyl donor, which is soluble in aqueous and organic solvents.
The highest selectivity was 95.5 with a conversion of 10%, whereas the higher conversion was 99% with selectivity of 70% after 4 h of reaction at 70
°C. Organo-chlorinated compounds were not found in products as in the case of the use of HCl as catalyst, which indicates that the intermediate
carbocation formed after alkene protonation is not susceptible to react with the chloroacetic anion. |
Well, that fits perfectly because we know that with HCl, in part terpenyl chloride [and possibly other chlorinated substitutes] is formed which is
precisely what we DON'T want. HCl is definitely a bad choice of catalyst here.
And it does indeed suggest that the trichloroacetate anion is far too soft a Lewis base to form an adduct with the carbocation. That's a good thing
because it allows water to do that job and form the carbinol.
And 'homogeneous acid catalysis' almost certainly means: with acetone. 
[Edited on 11-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The more I look at that paper (TCA, 2013) the less I like it. There are real ABSURDITIES in it.
| Quote: | | The catalytic tests were performed in a 100-cm3 three-necked-glass reactor with condenser and thermocouple. The reactor was submerged in thermostatic
bath with silicone oil and magnetic stirring. In batch experiment, 3.6 mmol of α-pinene, 18 mmol of water and 10 mL of aceton were first placed.
After heating to the desired temperature, 32 mol of the trichloroacetic acid catalyst was added. Aliquots were extracted with a micropipette and
immediately analyzed with GC. |
32 mol of catalyst!!! Of course that's a typo but which is it then: 32 mmol? 3.2 mmol? 32 micromol? We don't know!
| Quote: | | The formation of these compounds supports the above mentioned, and trichloroacetic acid could promote the water/pinene interaction, so protons at
organic phase promote mainly pinene rearrangement isomerization like in the isomerization process of pinene to produce camphene.
|
Tosh, balderdash and piffle: the system is uniphasic (all one phase: pinene, water, acetone and catalyst), there is no 'organic phase' here.
If there was, direct injection of sample into a GC would be very unreliable.
Finally, the yields mentioned aren't real yields, in the sense that no work-up was ever performed. These yields are simply GC values and thus really
optimistic.
I think a request for that other paper you mentioned should be made.
=======================
Some more information about acetone as a solvent here to follow later on...
[Edited on 11-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Notes on acetone as a solvent (here)
The need for a solvent has already been explained in terms of homogeneous catalysis being generally preferred.
Why then specifically acetone?
1. The solvent should be inert.
2. The solvent should be miscible with water and the reagents.
3. The solvent should preferably have a low BP.
Three candidates immediately spring to mind: acetone, MeOH and EtOH.
Unfortunately the latter two don't really comply to 1., as both are soft Lewis bases. The Wiki entry on alpha-pinene does indeed mention the formation
of terpine ethers in these solvents, by adduct formation with the carbocation.
Other possible candidates would be THF and ether but for obvious reasons acetone is to be preferred.
Acetone does show some reactivity towards water:
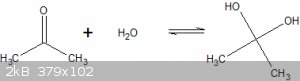
Propan-2,2-diol, aka a germinal diol, exists with acetone in equilibrium in a watery solution:
$$K=\frac{[\text{germinal diol}]}{[\text{acetone}]} \approx 10^{-3}$$
So that's about 1 in every 1000 acetone molecules in an acetone/water mixture is present as propan-2,2-diol.
(Source)
[Edited on 11-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by blogfast25  | The more I look at that paper (TCA, 2013) the less I like it. There are real ABSURDITIES in it.
...
32 mol of catalyst!!! Of course that's a typo but which is it then: 32 mmol? 3.2 mmol? 32 micromol? We don't know!
...
Tosh, balderdash and piffle: the system is uniphasic (all one phase: pinene, water, acetone and catalyst), there is no 'organic phase' here.
If there was, direct injection of sample into a GC would be very unreliable. |
Yes. 32 mol calculated out at something like 5 kilos or similar.
It must be mmol, seeing as it has to fit in a 100ml vessel.
Where the decimal point goes is anyone's guess.
The system isn't really uniphasic - if left unstirred two phases separate in a few seconds.
It does worry me that the three papers found, plus a great many more, could be entirely invented data.
When you think about it, Who would actually bother to test the process in a paper simply on a whim ? 10h stirring time etc - would it be worth it ?
It is quite possible that i simply cannot measure or stir things properly, nor count minutes and hours, but it seems a bit unlikely.
The chloral hydrate synth seems to be working fine by following the procedure found in Cohen, so i can't be ballsing up the other synths that
badly.
BTW: there are two RBFs with post-reflux layers in them waiting on any suggestions for an actual (workable) separation process.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
I. The system isn't really uniphasic - if left unstirred two phases separate in a few seconds.
II. It does worry me that the three papers found, plus a great many more, could be entirely invented data.
When you think about it, Who would actually bother to test the process in a paper simply on a whim ? 10h stirring time etc - would it be worth it ?
III. It is quite possible that i simply cannot measure or stir things properly, nor count minutes and hours, but it seems a bit
unlikely.
BTW: there are two RBFs with post-reflux layers in them waiting on any suggestions for an actual (workable) separation process.
|
I. Trust me, it is. It's 0.5 g pinene, 0.32 g water, 10 ml acetone and probably 0.52 g TCA. Pinene and TCA are both acetone soluble,
that bit of water won't cause problems.
II. I doubt if it's a fake. It's just a poor quality paper. Tons of research have been done on alpha-pinene/limonene to
alpha-terpineol.
III. No one's saying anything about 'ballsing things up'. Go higher up and you'll find me and 'ecclectic' trying to make
alpha-terpineol from turpentine. We failed. And we NEVER balls anything up! 
It could be the turps at fault but it doesn't look like it, see your distillation.
Not sure about those other org. phases. Have they been distilled yet?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
II, III Exactly.
It is just not that hard to reflux stuff, separate layers or distill, which is why i suspect that the actually useful parts of those experiments were
never done, relying instead on GC analysis to claim success.
If it needs a 20m column to separate the stuff, well, it's obviously useful in the understanding of the underlying chemistry, yet pointless if you
actually want to make a pot of turpineol juice.
Bought a litre and a half of brandy today to Assist in this research.
There was a mention in the Erowid archive of Hive (or is it the other way around ?) that chloral alcoholate needs distilling with conc sulph to get
the chloral, and that the ethanol needs to be rammed with Cl2 to get a decent yield.
Seems to work pretty well just dripping conc sulph onto the alcoholate, but it may well have volatilsed and buggered off before any water was added
We Will see.
No way this terpineol synth is going to get left behind.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
While we speculate and wait for darkness and drunken-ness to end, what actually happens when a substance like pinene dissolves in a Non-polar solvent
?
With water and sulphuric acid, the sulphuric spilts to H<sup>+</sup> and SO4<sup>2-</sup> and the water gets all
OH<sup>-</sup> and H3O<sup>+</sup>
What is the actual solvation mechanism for alpha-pinene and acetone ?
Presumably the species produced are reasons why the reaction needs acetone as a solvent.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
double post eliminated
[Edited on 11-1-2016 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Take HCl as a simpler example (monoprotic). As a strong acid it completely deprotonates:
HCl(aq) + H2O(l) === > H3O<sup>+</sup>(aq) + Cl<sup>-</sup>(aq)
So basically a 1 M HCl solution (for example) becomes a 1 M H3O<sup>+</sup> + 1 M Cl<sup>-</sup> solution,
or:
$$[\mathrm{H_3O^+}]=1\:\mathrm{M}$$
A second equilibrium in water is:
2 H2O(l) < === > H3O<sup>+</sup>(aq) + OH<sup>-</sup>(aq), with KW the
auto-dissociation constant of water:
$$K_W=[\mathrm{H_3O^+}][\mathrm{OH^-}]=10^{-14}$$
So that in acid conditions:
$$[\mathrm{OH^-}] \approx 0$$
alpha-pinene solvates in solvents like acetone (a polar solvent, BTW) without any such changes: it doesn't 'lose' bits and pieces to the solvent, like
acids do. Solvents like THF and diethyl ether would probably work too...
I'm inclined to give up on the old turps and switch to limonene instead. Similar chemistries but more selective.
[Edited on 11-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Er, No.
That is ducking the question and avoiding facing the problem head-on.
If they Dissolve and not simply Mix, Acetone and Pinene must React to form a solution containing the two species, and Other
intermediate species in some equilibrium.
The JPL paper gives data for acetone and sulphuric acid dissolving or reacting depending on acid concentration, so there's a clue.
I'm currently inclined to think that it is these intermediate species that affect the outcome so much that they need to be at least Known if not
Understood.
NOT doing so is the same as saying that H2SO4 reacts with pinene forming a carbocation without the acid even dissassociating.
That said, i have a lemon tree with a hell of a lot of lemons on it.
Edit:
I also have a litre and a half of Brandy 
[Edited on 11-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
I. If they Dissolve and not simply Mix, Acetone and Pinene must React to form a solution containing the two species,
and Other intermediate species in some equilibrium.
II. NOT doing so is the same as saying that H2SO4 reacts with pinene forming a carbocation without the acid
even dissassociating.
III. That said, i have a lemon tree with a hell of a lot of lemons on it. |
I. Sigh, sigh and double sigh... Aga, mixing and dissolving are
the same thing here. If you mix two substances that are miscible then by definition they form a solution that is of course by definition a
one phase system. That is NOT a reaction though. Dissolving sugar in water (e.g.) is not a reaction. Solvation should not be equated to reaction, even
though they have some things in common. Aga, mixing and dissolving are
the same thing here. If you mix two substances that are miscible then by definition they form a solution that is of course by definition a
one phase system. That is NOT a reaction though. Dissolving sugar in water (e.g.) is not a reaction. Solvation should not be equated to reaction, even
though they have some things in common.
II. Sorry: gibberish to me. In some aprotic solvents and specific strong acids that might even work here. But here we need
the water for the OH addition, to get the carbinol.
III. Limonene is too OTC to start steam distilling citrus peels.
Changing precursor is nothing shamefull. But flogging a dead horse is a waste of time... 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK. So i need to have a good long stare at dissolution i guess.
Got turps. Not got limonene.
ISTR that it's a major product when pyrolysing old rubber tyres ...
Can't give up just yet - today there are chloral hydrate crystals.
[Edited on 12-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | OK. So i need to have a good long stare at dissolution i guess.
Got turps. Not got limonene.
ISTR that it's a major product when pyrolysing old rubber tyres ...
Can't give up just yet - today there are chloral hydrate crystals.
[Edited on 12-1-2016 by aga] |
I'll see if I can rustle something up with regards to solvation. It does of course play a part: two same reactions carried out in two different inert
solvents often show different reaction rates, so something is going on there.
Why not finish the synth. of the TCA if you can, then try alpha-pinene in GAA with TCA as catalyst? That should give alpha-terpineol acetate (plus
cruds).
I've become very pessimistic about this whole endeavour: driving blind without GC/IR makes it very difficult to find that 'sweet
spot' of temperature and cooking time.
I was looking (in another thread) at hydrochlorination of limonene with HCl(g) to alpha-terpinyl chloride but the problem is similar: unknown reaction
times and by-products.
******************
Oh, and I found a better version of the '2013, TCA' paper, this time as an improved conference paper:
http://psrcentre.org/images/extraimages/13%201212598.pdf
At least the procedure now seems correct. And it does include a direct comparison of TCA and H2SO4, showing that TCA works
better.
| Quote: | | The catalytic tests were performed in a 100-cm3 threenecked-glass reactor with condenser and thermocouple. The reactor was submerged in thermostatic
bath with silicone oil and magnetic stirring. In batch experiment, 0.25 mol of α-pinene, 0.6 mol of water and 20 mL of aceton were first placed.
After heating to the desired temperature (70o C), 0.11 mol of the catalyst was added. Aliquots were extracted with a micropipette and immediately
analyzed with GC. Conversion (X %) was defined here as moles of monoterpene converted per 100 moles of monoterpene feed. The selectivities of
α-terpineol (S %) were defined as moles of α-terpineol formed per 100 moles of α-pinene converted. |
That's 34 g alpha-pinene, 10.8 ml water, 18 g of TCA and 20 ml of acetone.
Fig. 2 shows the selectivity and suggests a reflux time of about 45 minutes to be optimal. Dixit the authors, of course. 
In your case the cooled (force cooled if possible to control thermal history better) mixture would have to be neutralised immediately with NaOH to
prevent further hydration of any alpha-terpineol formed.
Then add 100 ml of salt brine (saturated NaCl) and shake'n vent several times. Organic phase should separate to the top. Separate and shake'n vent
with water. Separate organics and distil off low boilers. May the force be with you!
[Edited on 12-1-2016 by blogfast25]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Try stirring a solution of pinenes or limonene in acetic acid in the presence of 1 mol% conc. sulfuric acid. A sulfonic acid such as MsOH and TsOH, or
sulfamic acid, would be preferable over sulfuric acid, but the choice depends on availability. Do not overload the acid and follow the reaction by TLC
- it should be relatively fast at rt and should give terpinyl acetate as the major product. Going directly to the alcohol is certainly not as trivial
and selective.
A synthesis of terpinyl acetate from pinenes and limonene using CAN on silicagel is described in DOI: 10.5650/jos1956.38.553. CAN is quite a common
reagent. Alternatively, one could consider ZnCl2 on silicagel (easily prepared by rotavaping a slurry of silicagel in methanolic
ZnCl2).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@Nicodem:
Thanks. The de-esterification of terpineol acetate won't be easy either though, I imagine.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
The Farce is with us young BlogWalker.
Therefore We cannot fail : Keep the Faith.
Excellent ! Some actual data to work with ! Woohoo !
Nice find.
Quote: Originally posted by Nicodem  | | Try stirring a solution of pinenes or limonene in acetic acid in the presence of 1 mol% conc. sulfuric acid. A sulfonic acid such as MsOH and TsOH, or
sulfamic acid, would be preferable over sulfuric acid |
Not looked up what MsOH or TsOH are, however there does seem to be an unopened bottle of sulphamic acid on the shelf.
'acetic acid' ?
Does that mean glacial acetic acid or would vinegar do ?
P.S.
The Brandy has almost all gone : it's heading the right way for a whole pile of TCA.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Excellent ! Some actual data to work with ! Woohoo !
Nice find.
Quote: Originally posted by Nicodem  | | Try stirring a solution of pinenes or limonene in acetic acid in the presence of 1 mol% conc. sulfuric acid. A sulfonic acid such as MsOH and TsOH, or
sulfamic acid, would be preferable over sulfuric acid |
Not looked up what MsOH or TsOH are, however there does seem to be an unopened bottle of sulphamic acid on the shelf.
'acetic acid' ?
Does that mean glacial acetic acid or would vinegar do ?
The Brandy has almost all gone : it's heading the right way for a whole pile of TCA.
|
GAA = pure acetic acid. It's what he meant. Vinegar is only good for fish and chips.
TsOH: ortho-toluenesulphonic acid.
You are the Keith Floyd of amateur OC and the Keith Moon of casual alcoholism!   
[Edited on 12-1-2016 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Such Compliments i am certainly unworthy of !
--------------------------------------------------------
Sorry for the delay,
lack of absolute EtOH has eaten a day.
Distilling many litres like mad,
the smell was still Bad.
So distilled it some more,
to even the score.
In went the K2CO3, so the water is spent,
Anhydrous it Is, or near 99 percent.
So 200ml was subjected to chlorination,
It has to be said - with some trepidation.
With all in place and gas mask on face.
onto the TCCA went the HCl, apace.
Hubbles and bubbles and Cl2 around,
it rushed through the EtOH with no precipitate found.
Changing the tack,
the inverted funnel was put back.
Faster and faster the Cl2 rushed though,
reacting and heating, if only i knew !
Quick ! Quick ! Put in a Bath !
it was so farcical, one could but laugh.
There it remains, the night not yet ended,
no chemistry goes quite how one intended.
[Edited on 13-1-2016 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You do realise that the penitence for successfully synthing TCA... is a proper write-up? 
|
|
|
| Pages:
1
..
12
13
14
15
16
..
19 |