| Pages:
1
2 |
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by AJKOER  |
True, but lab light appears to produce remarkable decomposing ability on some sulfides, some of which that appear to be seemingly impervious to even
pure oxygen treatment alone (hence the use of a combined O2/light protocol).
See, for example, commentary on Cadmium sulfide under lab light at https://books.google.com/books?id=GClzU2-Rj18C&pg=PA487&... with reports in some labs of 50% to 90% decomposition! The effect of the light is
described as 'immediate' and 'variable'.
|
it is even cited on wikipedia, but cadmium sulphide is the catalyst to the decomposition of hydrogen sulphide in solution, it doesn't decompose itself
(it was used as a pigment in the 1800, so if it was that unsable it would have never been used as a pigment...) and it does not decompose any
sulphide, only hydrogen sulphide is mentioned, on wikipedia is even proposed a mechanism
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Ubya  | Quote: Originally posted by AJKOER  |
True, but lab light appears to produce remarkable decomposing ability on some sulfides, some of which that appear to be seemingly impervious to even
pure oxygen treatment alone (hence the use of a combined O2/light protocol).
See, for example, commentary on Cadmium sulfide under lab light at https://books.google.com/books?id=GClzU2-Rj18C&pg=PA487&... with reports in some labs of 50% to 90% decomposition! The effect of the light is
described as 'immediate' and 'variable'.
|
it is even cited on wikipedia, but cadmium sulphide is the catalyst to the decomposition of hydrogen sulphide in solution, it doesn't decompose itself
(it was used as a pigment in the 1800, so if it was that unsable it would have never been used as a pigment...) and it does not decompose any
sulphide, only hydrogen sulphide is mentioned, on wikipedia is even proposed a mechanism |
The CdS in paint is not photo-active polycrystalline CdS, and sunlight is not identical to lab light, as apparently CdS is photo-active to select wave
lengths of light. Here is a study (link https://aip.scitation.org/doi/abs/10.1063/1.330618 ), to quote:
"The mechanism of photoconductivity in polycrystalline CdS has been studied over the temperature range 100–300 K using Hall‐effect and
conductivity measurements in the dark and under white light illumination....The detailed variations of μ and n̄ are interpreted in terms of the Seto
model with the added hypothesis that photogenerated holes...."
The creation of electron holes (h+) means in the present of say water vapor:
H2O <--> H+ + OH-
the OH- ion becomes an hydroxyl radical (•OH), and HS- ion becomes the •HS radical which may further react with oxygen creating the powerful
reducing radical, •SO2-, as detailed above.
With photo-generated electrons (e-), H+ becomes the hydrogen atom radical. (•H), oxygen in air becomes the superoxide radical anion....
But, you are correct that light itself does not change CdS as h+ cancels e-. There is, nevertheless, possible disruption to intended preparations and
associated chemical reactions with the CdS from photo created products.
This is likely implied in my referenced ebook, to quote:
"5.1.2 Cadmium sulfide is not appreciably oxidized even when aspirated with pure oxygen in the dark. However, exposure of a bubbler containing cadmium
sulfide to a laboratory or to more intense light sources produces an immediate and variable photo decomposition. Losses of 50 to 90% of added sulfide
have been routinely reported by a number of laboratories.”
[Edited on 22-7-2019 by AJKOER]
[Edited on 22-7-2019 by AJKOER]
|
|
|
icelake
Harmless

Posts: 48
Registered: 9-10-2017
Member Is Offline
Mood: No Mood
|
|
| Quote: | | "5.1.2 Calcium sulfide is not appreciably oxidized even ..." |
It's Cadmium sulfide not Calcium sulfide.
and more importantly, what does CdS (insoluble in water & used in photoresistors) have anything to do with Na2S?
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by icelake  | | Quote: | | "5.1.2 Calcium sulfide is not appreciably oxidized even ..." |
It's Cadmium sulfide not Calcium sulfide.
and more importantly, what does CdS (insoluble in water & used in photoresistors) have anything to do with Na2S?
|
Thanks, my advanced logic talk-to-text software I used to reproduce the extracted text from the ebook made a substitution. Apparently, healthy calcium
is a much more commonly referenced than toxic cadmium.
I have corrected it in my extract.
-----------------------------------------
Per this extract from 2018 at https://pubs.rsc.org/en/content/articlelanding/2018/cc/c8cc0... :
"shows highly selective photooxidation of sulfides"
implies to me that sulfides, in general, may have associated photo sensitivity properties. Iodide salts also have varying light sensitivity.
Work on sensor development (see https://www.mdpi.com/1424-8220/16/3/296/htm ) suggest the use of tin sulfide, in particular, and states more generally:
"The results highlighted the possibility to use metal sulfides as a novel class of sensing materials, owing to their selectivity to specific
compounds, stability, and the possibility to operate at room temperature."
This source (https://materion.com/resource-center/product-data-and-relate... ) notes more generally:
"Some sulfides possess semi-metallic character and have potentially valuable electronic properties."
---------------------------------------------------------
Interestingly, a web search cites Wiki as a source claiming that CdS is "sensitive to visible and near infrared light". An MSDS to quote (source: https://www.fishersci.com/store/msds?partNumber=AC310180100&... ):
"Stability - Stable under normal conditions. Hygroscopic. Air sensitive. Light sensitive.
Conditions to Avoid - Exposure to air. Exposure to light. Incompatible products. Exposure to moist air or water."
The current Wikipedia (https://en.wikipedia.org/wiki/Sodium_sulfide ) on Na2S notes oxygen sensitivity, so per Wiki a combination of O2/UVC is not even needed for its
decomposition.
Also, as to mechanics for decomposition of the pure product, since aqueous Na2S is alkaline, and given the photo sensitivity, albeit low, of the OH-
ion (to UV):
OH- + hv --UV Light--> •OH + e-
as per a source ( https://pubs.rsc.org/en/content/articlelanding/2017/cp/c7cp0... ) to quote: "Irradiation by the light at the Urbach tail excites an electron out
of the hydroxide ion, leaving a neutral OH radical behind." Then:
•OH + H2S --> H2O + •HS (Source (link fixed) at p. 7, Eq (9) at https://pdfs.semanticscholar.org/533e/9a0b2e5d938abc555e267f... )
•HS/•S- + O2 --> •SO2- (+ H+) (Source (link fixed) at p. 7, Eq (7) at https://pdfs.semanticscholar.org/533e/9a0b2e5d938abc555e267f... )
which produces but a limited contribution, in my opinion, to so called 'oxygen sensitivity'. In fact, as one source notes, there is no significant O2
reaction until the creation of colloidal sulfur (with the presence of oxygen and say an iron impurity, or e- , generated from the sulfur's colloidal
electrostatic charge, or light,..). Then, with associated polysulfides, in the presence of solvated electrons, possible creation of the very light
sensitive colorful sulfur radical anions, •Sn-, where n= 2,3 and 4 (see https://pubs.acs.org/doi/abs/10.1021/ic50189a042 ). These colorful sulfur radicals likely, per a modified Eq (7) above, then could lead to •SO2-
formation as follows:
•Sn- + O2 --> •SO2- + Sn-1
Ultimately as previously detailed above, all per very recent cited work (2017, 2019,..), it is possible for •SO2- to form S2O4 (2-), which undergoes
acid hydrolytic disproportionation to thiosulfate and bisulfite, which are cited decomposition products.
[Edited on 22-7-2019 by AJKOER]
|
|
|
icelake
Harmless

Posts: 48
Registered: 9-10-2017
Member Is Offline
Mood: No Mood
|
|
The highlights:
| Quote: | | Recently, coordination polymers (CPs) have opened up remarkable performance in the growing field of photocatalysis, which is primarily benefited from
the apt incorporation of various chromophores and/or functional groups in organic linkers. |
| Quote: | | We describe herein a novel visible-light-photoactive coordination polymer for triggering selective aerobic transformation of sulfides to generate
sulfoxides. Orange strip-shaped crystals of 1 were prepared by a solvothermal reaction of
Zn(NO3)2.6H2O and 4,40-(anthracene-9,10-diylbis(ethyne-2,1-diyl))dibenzoic acid (ADBEB) in a mixed solvent of
H2O/CH3CN/DMA (DMA =N,N-dimethylacetamide). 1 can also be synthesized in the mixed solvent of
H2O/DMA with a lower quality and yield, but cannot be synthesized using DMA solely. |
| Quote: | | The capability of 1 serving as a visible-light photocatalyst to carry out aerobic oxidation of sulfides was then investigated, where a typical sulfide of thioanisole (PhSMe) was used as a model substrate and the conversion of thioanisole was
monitored via gas chromatography-mass spectrometry (GC-MS). |
| Quote: | | The use of zinc nitrate, i.e. the metal salt for the synthesis of 1, as the catalyst cannot initiate the reaction, which indicates
that the photocatalytic activity of 1 results from the organic linker. |
TLDR: Photooxidation of R-S-R to R-S=O-R using 1 as a photocatalyst. So much in common with the
current discussion (Sodium sulfide), amiright?
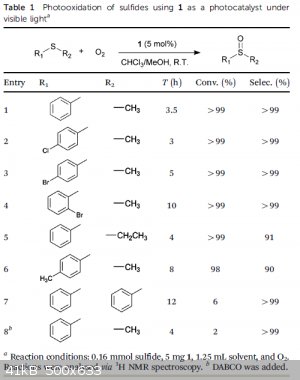
Attachment: Selective photooxidation of sulfides mediated by singlet oxygen using visible-light-responsive coordination polymers.pdf (2MB)
This file has been downloaded 301 times
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I was skeptical a bit about this but have changed my mind. Still not sure how to control the temperature, but the dry way of connecting sulfur with
the Na cation obviously works.
I did this experiment on the weekend:
2 SnO2 + 2 Na2CO3 + 9 S = 3 SO2 + 2 Na2SnS3 + 2 CO2
Works like a charm, and I had only an alcohol burner to make this.
What I don't like both in my experiment and also in your suggestion.
My impression was that this s$1t also emits a good portion of CO - 2 days of headache. So, be careful.
And what is most interesting for me, is the information I red today in some old book that probably almost any Na2S becomes contaminated with Na2CO3 -
by this reason it is not possible to use Na2S as a source of H2S for the purpose of qualitative analysis (unlike H2S from FeS it will be always
contaminated with CO2 according to the author).
[Edited on 22-7-2019 by teodor]
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  |
And ofcourse everything is in some sort of a equilibrium, if enough methanol is added at some point almost pure NaOCH3 would be left wouldn't it? If
above is correct.
[Edited on 18-7-2019 by Tsjerk] |
You are right, the experiment was mentioned in the paper did the study of solution composition after hours of keeping it under reflux. So, it is
obviously some state of equilibrium and they didn't try to study the actual process.
But, the equilibrium should be shifted because NaHS is much less soluble in alcohol.
Also, see my previous note about Na2CO3 contamination.
My personal experience with boiling Na2S2O3 and S in the presence of ethanol: H2Sx (sulfanes) were formed, it could be evident because on cooling S
was deposited in the condenser (I found this is a property of sulfane reacting with a glass). So, there is also gas phase and they didn't study it.
[Edited on 22-7-2019 by teodor]
|
|
|
icelake
Harmless

Posts: 48
Registered: 9-10-2017
Member Is Offline
Mood: No Mood
|
|
Apparently highly pure sodium sulfide can be produced by electrolysis, but it's not an amateur friendly process (GB470033A).
| Quote: | | It can also be produced by electrolysis. The latter product is far more pure. |
Note: CA230269A and US1502213A are the same.
Attachment: Industrial Uses of Sulfur and Its Compounds.pdf (577kB)
This file has been downloaded 335 times
Attachment: CA230269A.pdf (302kB)
This file has been downloaded 239 times
Attachment: US1502213A.pdf (239kB)
This file has been downloaded 253 times
Attachment: GB470033A.pdf (164kB)
This file has been downloaded 271 times
|
|
|
| Pages:
1
2 |
|