DReed123
Harmless

Posts: 8
Registered: 23-6-2019
Member Is Offline
|
|
Preparation of Effexor
I take this medication (Venlafaxine/Effexor: https://en.wikipedia.org/wiki/Venlafaxine), and having wrapped up two semesters of Organic Chem thought it would be fun to try and design a
synthesis for it. I asked this on stackexchange and someone pointed out my initial attempt would cause an unexpected intramolecular ring closure. I'm
wondering if this reordering of steps would work:
Beginning with Anisole
1.Friedel-Crafts alkylation: allyl bromide (3-bromo-1-propene) and AlBr3 adds propene to the para position.
2 .Radical bromination should be selective for the benzylic position.
3. Grignard reaction with cyclohexanone.
4.Ozonolysis with DMS
5.Reductive amination with dimethylamine and triacetoxyborohydride.
Any feedback would be much appreciated.
|
|
|
ZetekitoxinAB
Harmless

Posts: 19
Registered: 31-7-2019
Member Is Offline
Mood: Omberacetam
|
|
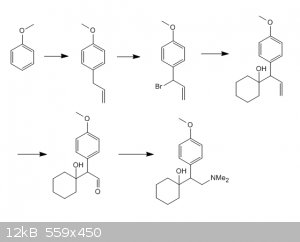
Hi ! I see many potential problems with your proposed synthesis.
1. Allyl bromide would definitely give a mixture of hard to separate o- and p- isomers. Even if you used stoechiometric amounts, I assume
polisubstitution would likely occur, as the methoxy group of the anisole is very activating. Much better practically would be to acylate the ring with
acryloil halide, separate the o- p- isomers and reduce the carbonyl via hydrazine+KOH (Kishner Wolff reduction).
2. I am not sure the Grignard derived from the bromo- compound 3 would be stable without isomerisation, reacting with cyclohexanone on the end of the
chain instead.
3. I would doubt you could selectively ozonize compound 4; again the methoxy group increases the reactivity of the ring towards ozone and also the
tertiary alcohol should be protected before ozonolysis. You would have to propose another cleavage route of the double bond to aldehyde.
[Edited on 4-8-2019 by ZetekitoxinAB]
[Edited on 4-8-2019 by ZetekitoxinAB]
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
As pointed out in the post above mine, your proposed route is a recipe for heartache and synthesis disaster. Did you do a lit search on the synthesis
of venlafaxine as a starting point for alternative routes? Two patents detail the commercial routes both of which you should be able to get with a
google search. To wit:
EP 112669 (1984)
GB 227743 (1989)
The reported syntheses are much more straightforward and more chemically sensible than what you have proposed.
Both of these have prior US filings so there may also be a US patent.
AvB
|
|
|
DReed123
Harmless

Posts: 8
Registered: 23-6-2019
Member Is Offline
|
|
Thanks to both of you for taking the time to respond. I didn't do any research regarding its actual synthesis as it was intended to be a challenge for
myself to figure out. Wasn't interested in efficiency so much as it just being correct. That's interesting regarding aryls being vulnerable to
ozonolysis...didn't know that.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
starting from 4-methoxyphenyl oxirane ( CAS no: 6388-72-3)
1.reaction with dimethylamine
2.conversion of benzylic alcohol to chloride using TCT
3.grignard reaction with cyclohexanone
Quote: Originally posted by ZetekitoxinAB  | | I would doubt you could selectively ozonize compound 4; again the methoxy group increases the reactivity of the ring towards ozone
|
You can.See ozonolysis of eugenol - https://en.wikipedia.org/wiki/Ozonolysis#Ozonolysis_of_alken... | Quote: | | also the tertiary alcohol should be protected before ozonolysis. |
ozone converts t-OH's to
hydroperoxides,which can be hydrolysed back to alcohols,so no protection is required
[Edited on 4-8-2019 by CuReUS]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I would envisage something like
1) clasien condensation of dimethylacetamide with cyclohexanone, somehow preventing dehydration or doing the hydration in a seperate step directly
afterwards.
2) arylation of the alpha position of the amide with 4-bromoanisole
3) complete reduction of the amide
Single biggest problem with this will be dehydration and then it will become the alkane instead.
Another way that comes to mind is condensation of 4-methoxyphenylacetonitrile with cyclohexanone, again avoiding dehydration. Then a two step one pot
reduction/reductive amination with formaldehyde to the product.
Cureus, I haven't checked but are gringard reactions possible on nitrogen mustards? I think there is quite some potential for polymerization.
|
|
|