Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
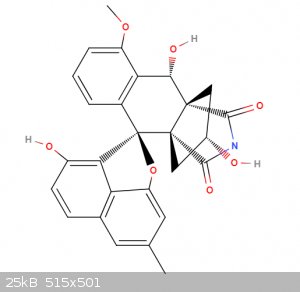
Lugdunomycin
Look at this beauty! A completely novel structure found not to long ago. Named after the city I live. My girlfriend was one of the people who found it

"Lugdunomycin has unique structural characteristics, including a heptacyclic ring system, a spiroatom, two all-carbon stereocenters, and a
benzaza-[4,3,3]propellane motif"
Article
Attachment: MolView.mol (5kB)
This file has been downloaded 584 times
At least now I can't use it in the guess the compound game anymore 
[Edited on 23-8-2019 by Tsjerk]
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
I had to look up what a spiroatom was: the only common atom of two rings.
Is there a special significance to it having a spiroatom?
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Just speculating, but it could be relevant as it makes the quite extreme 3D structure possible.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Wow, that looks like a beast.
Anyone up for a total synthesis? 
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
thought you would never ask 
My method ( sans stereo  ) )
1.thermal rearrangement of molecule A
2.diels alder with molecule B
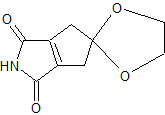
3.demethylation of phenol using BBr3
4.double oxidation of the benzylic positions using SeO2
5.protection of phenol using TIPS (Q)
6.conversion of the bottom ketone to gem-dibromide using PBr5 - https://www.embibe.com/study/reaction-of-aldehydes-and-keton...
7.Modified corey house reaction with molecule C
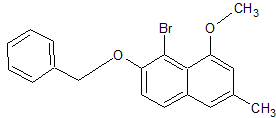 | Quote: | | From the stoichiometry, it is apparent that one equivalent of the R group is wasted as an ill-characterized alkylcopper species (likely polymeric;
usually converted to RH upon aqueous workup) in the most common form of the Corey–House synthesis. To avoid this for cases where R is a precious or
complex fragment, a reagent (R)(RU)CuM, where RU is an untransferable dummy ligand (e.g., RU = cyano, alkynyl, 2-thienyl, etc.) can be prepared and
used instead. |
https://en.wikipedia.org/wiki/Corey%E2%80%93House_synthesis#...
8.demethylation of phenol using BBr3 followed by williamson ether synthesis
9.removal of TIPS(Q) using nBu4NF- ,followed by methylation
10.debenzylation using H2/Pd/C
11.glycol hydrolysis to reveal the cyclopentanone
12.double NaBH4 reduction of ketones to get the target compound
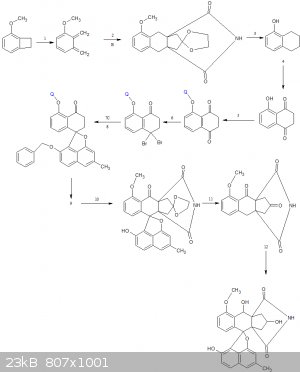
[Edited on 24-8-2019 by CuReUS]
|
|
|
Carbon8
Harmless

Posts: 34
Registered: 1-1-2018
Member Is Offline
Mood: No Mood
|
|
Lugdunomycin versus Lugdunin: Why would the discoverers of a potential antibiotic choose a name that's so similar to another recently discovered
antibiotic?
https://en.wikipedia.org/wiki/Lugdunin
https://www.nature.com/articles/nature18634
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
The names seem similar, but are different.
about Lugdunin (or actually the species producing it):
"It was first described in 1988 after being differentiated through DNA analysis. Its name comes from Lugdunum, the Latin name for Lyon, France, where
the organism was first isolated."
The name for Leiden, Netherlands is Lugdunum Batavorum, which means as much as Lyon of the Bataviers as it was named after Lyon by the Romans.
about Lugdunomycin: The Lugdun part is equal, but the ending is a reference to the species it was extracted from. Streptomycin is famous for it's
name; the -mycin ending refers to it being a fungus. It is not. Decades after naming it like a fungus it was found it is actually a bacterial species
which looks and behaves like a fungus.
I love your post! I will take some time soon to have a good look at it 
[Edited on 25-8-2019 by Tsjerk]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
modified 3 step method 
1.williamson ether synthesis with molecule A
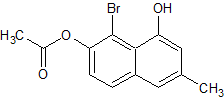
2. https://onlinelibrary.wiley.com/doi/abs/10.1002/chem.2017006...
3.diels alder with molecule B
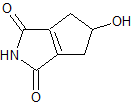
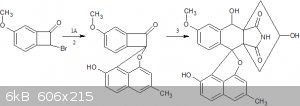
[Edited on 26-8-2019 by CuReUS]
|
|
|