Quieraña
Harmless

Posts: 34
Registered: 24-8-2019
Member Is Offline
|
|
Cubane instead of benzene with the phenylethylamine backbone
Now I get that this topic touches a little bit if not a lot on dear taboo subject matter given phenylethylamines and what bad things can come of that.
That being acknowledged I am strictly curious first of all if one were to have cracked a synthesis for making cubane at home is it possible to
substitute it in the phenylethylamine skeleton? If so has anyone ever heard of such a thing? Can you imagine what that might bring to Medicine? I
don't assume that they would strictly be stimulants but if it were I can only imagine it would be perhaps six times stronger but then again I'm
thinking only because it's a cube and not a two-dimensional ring. Nevertheless for the past 6 months it has tantalized my imagination as to its
possibilities assuming it's not a poison and worthwhile in medicine.
[Edited on 25-8-2019 by Quieraña]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Your idea is indeed promising. See here:
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.2015106...
https://blogs.sciencemag.org/pipeline/archives/2016/02/09/cu...
https://pubs.rsc.org/en/content/articlelanding/2019/ob/c8ob0...
The tricky part is making the cubane.
|
|
|
Quieraña
Harmless

Posts: 34
Registered: 24-8-2019
Member Is Offline
|
|
Merry christmas, you are awesome for finding those. Thank you.
|
|
|
brubei
Hazard to Others
  
Posts: 187
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
try to find cubane building block seller like
https://enamine.net/building-blocks/medchem/view-all/cubane-...
I'm French so excuse my language
|
|
|
Tellurium
Hazard to Self
 
Posts: 84
Registered: 12-7-2017
Location: Group 16, Chalcogen City
Member Is Offline
Mood: smelly
|
|
Well synthesis of the cubane itself seems doable, but it requires quite some steps and especially a lot of reagents:
https://www.synarchive.com/syn/14
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
That looks pretty OTC! If one could do the first step in DCM instead of CCl4 and have SOCl2 on hand it is doable if you just aim on getting to the
cubane backbone. I think loosing the carboxylic functional group is a waste anyway.
One only needs to have a source of potassium terr-but oxide, but that shouldn't be that much of a problem.
Acces to a NMR would also help, apparently the spectrum is very specific.
[Edited on 28-12-2019 by Tsjerk]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
You'll find this synthesis interesting then... As far as otc goes, Check scheme 5.
I've got no idea how you'd translate the 2,5-dimethoxy pattern onto cubane or how you'd even introduce substituents onto cubane. Perhaps you can do
some directed metalations but that's about it I think.
Attachment: falkiner2013.pdf (1.4MB)
This file has been downloaded 322 times
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
god i wish i lived in a world where i could experiment with replacing benzene rings with 3D structures. someday...):
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
It looks surprisingly doable, could be a fun project. Carbon tetrachloride is available otc in dry cleaning and break cleaning products. Thionyl
chloride is perfectly doable if you have the equipment, the only problem I see is the t-butyl organ. peroxide, and I’m sure you could figure that
out.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I think I have a new project on hands...
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
I like this idea but im skeptical of cubanes ability to substitute benzene of all things due to it lacking any kind of resonance.
If we wanted to substitute cubane then we really dont need to look much further than the already planned synthesis.
After rearrangement of the final halo ketone, we are left with a carboxylic acid which would be our starting point for further substitutions.
For a phenethylamine backbone the carboxylic acid could be reduced to an aldehyde and then react this with hydrogen cyanide to form a hydroxynitrile.
Followed by a reaction with thionyl chloride to form a halonitrile followed by a reaction with excess ammonia or the Gabriel synthesis and finally
hydrolysis to form an amino acid.
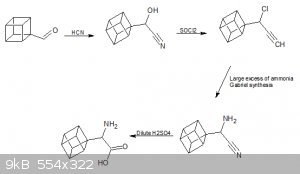
This would be a good starting point for any other modifications we would like to make but i think just getting to an amino acid would be successful.
https://www.jstage.jst.go.jp/article/bcsj1926/63/8/63_8_2397...
With all this said, this is multi step organic synthesis and every step has a chance of something going wrong as well as decreasing our final yields
drastically.
This is quite difficult practical research even for a well established laboratory with experience in this kind of thing.
Sufficiently advanced science is indistinguishable from madness.
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Quote: Originally posted by Tsjerk  |
That looks pretty OTC! If one could do the first step in DCM instead of CCl4 and have SOCl2 on hand it is doable if you just aim on getting to the
cubane backbone. I think loosing the carboxylic functional group is a waste anyway.
One only needs to have a source of potassium terr-but oxide, but that shouldn't be that much of a problem.
Acces to a NMR would also help, apparently the spectrum is very specific.
[Edited on 28-12-2019 by Tsjerk] |
If you use DCM as a solvent, remember that it can ignite in contact with the potassium t-butoxide.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by Abromination  | Quote: Originally posted by Tsjerk  |
That looks pretty OTC! If one could do the first step in DCM instead of CCl4 and have SOCl2 on hand it is doable if you just aim on getting to the
cubane backbone. I think loosing the carboxylic functional group is a waste anyway.
One only needs to have a source of potassium terr-but oxide, but that shouldn't be that much of a problem.
Acces to a NMR would also help, apparently the spectrum is very specific.
[Edited on 28-12-2019 by Tsjerk] |
If you use DCM as a solvent, remember that it can ignite in contact with the potassium t-butoxide. |
I think you're confusing t-butyl peroxide with potassium t-butoxide. In the linked synthesis, potassium t-butoxide is not used.
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Quote: Originally posted by Metacelsus  | Quote: Originally posted by Abromination  | Quote: Originally posted by Tsjerk  |
That looks pretty OTC! If one could do the first step in DCM instead of CCl4 and have SOCl2 on hand it is doable if you just aim on getting to the
cubane backbone. I think loosing the carboxylic functional group is a waste anyway.
One only needs to have a source of potassium terr-but oxide, but that shouldn't be that much of a problem.
Acces to a NMR would also help, apparently the spectrum is very specific.
[Edited on 28-12-2019 by Tsjerk] |
If you use DCM as a solvent, remember that it can ignite in contact with the potassium t-butoxide. |
I think you're confusing t-butyl peroxide with potassium t-butoxide. In the linked synthesis, potassium t-butoxide is not used.
|
Oh, you are right, tsjerk mentioned the K t-butoxide and that must have been what I was thinking.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Ah, yes, I thought potassium tert-butoxide was used, tert-butoxide peroxide is going to be a bit harder to acquire. Not undo-able though.
I'm not thinking about substituting a phenyl group in any molecule, I just want to make the cubane backbone. Just getting a cubane NMR spectrum would
be wonderful!
I'm looking into this
Edit: I'm pretty sure I could get someone crazy enough to do a NMR if I could convince them I made cubane...
[Edited on 30-12-2019 by Tsjerk]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I'm thinking:
Adipic acid + barium hydroxide = cyclopentanone
cyclopentanone + ethylene glycol = Cyclopentanone ethylene ketal
1,4-dioxane* has been done and reported about here.
Methanol, H2SO4, NaOH is all OTC. Bromine is easy as well.
Ba(OH)2 I have, so I would only need adipic acid and a proper distillation setup. Also an UV LED would be needed, but those come cheap nowadays.
* Nevermind, I can buy it.
[Edited on 30-12-2019 by Tsjerk]
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Quote: Originally posted by Tsjerk  | I'm thinking:
Adipic acid + barium hydroxide = cyclopentanone
cyclopentanone + ethylene glycol = Cyclopentanone ethylene ketal
1,4-dioxane* has been done and reported about here.
Methanol, H2SO4, NaOH is all OTC. Bromine is easy as well.
Ba(OH)2 I have, so I would only need adipic acid and a proper distillation setup. Also an UV LED would be needed, but those come cheap nowadays.
* Nevermind, I can buy it.
[Edited on 30-12-2019 by Tsjerk] |
NileRed i think has a video on adipic acid, if not I’m sure he has mentioned a source. He used it quite often. What is your source of barium?
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Presumably barium carbonate from pottery suppliers. I'd be willing to bet the reaction also chooches with that though.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I got a couple of boxes full of chemicals someone had to get rid of over a decade ago, there was some barium hydroxide in there. No idea of the
purity, but is a fine white powder.
Adipic acid is sold by eg. Labstuff.nl, he also sells dioxane.
Edit: now I think of it.. I got the boxes full of chemicals from Labstuff in the time before his webshop.
[Edited on 31-12-2019 by Tsjerk]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
This is pretty cool.
I wonder if Ladenburg benzene would act similarly?
Probably even harder to make though.
EDIT: apparently most folks call it prismane.
[Edited on 1-1-2020 by SWIM]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I ordered 1,4-dioxane and cyclopentanone, cubane coming up!
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Cyclopentanone is somehow more aggressive towards living tissue than say cyclohexanone! Severe eye irritation comes to mind. Careful!
|
|
|