chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
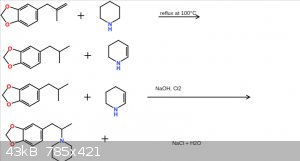
piperidine safrole additive
okay, ive been wanting to make a synth on this safrole derivitive for a while, but im not 100% sure if this particular route would work. what do you
guys think? suggestions are appreciated!
[Edited on 13-9-2019 by chemist1243]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
For me this needs a bit more context. Why do you think could work? It would be nice if it does, but how?
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
I'm with Tsjerk on this.
What's the mechanism?
And how about the safrole derivative you're starting with?
How do you make that?
And what happens to that disappearing carbon atom?
Where does it go?
That final compound looks familiar. Is it in Pihkal somewhere?
If so, that might ne a good place to start.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
What I'm seeing looks like MDMA with the n, methyl replaced by piperidine correct?
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Yes, nice looking molecule, but I don't think it is psychoactive. 3,4-Methylenedioxy-N,N-dimethylamphetamine is already hardly active, and this
secondary amine is even more shielded.
I had a quick look at this reaction scheme and I think it doesn't make sense. What is this starting molecule?
If you really want to make this compound just react isomerized safrole with hydrobromic acid, make the ketone and do a standard reductive amination
with piperidine.
If you really have this compound and you want to turn the 2-methyl into an amine, you will have a problem. Selectively breaking C-C bonds isn't easy.
[Edited on 14-9-2019 by Tsjerk]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Starting compound looks like the ketone just looks like they left the O off the molecule.rxn scheme looks wrong with NaOH and chlorine.very wrong.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by Tsjerk  |
If you really want to make this compound just react isomerized safrole with hydrobromic acid, make the ketone and do a standard reductive amination
with piperidine. |
I'd say, even easier, react bromosafrole with piperidine.
Also, I suppose it could be far more psychoactive, when we think of relatively similar compounds like MDPV.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Okay, so I see some of you are confused. First of, this reaction scheme was made in sigma Aldrich, and I cropped out the bottom so it looks like the
piperidine is missing a carbon, it is not. The precursor is safrole, not safrole oxidized to a ketone. I should also mention that instead of just one
mole of NaOH being reacted with cl2, 2 moles of NaOH will be used. This is because 2NaOH + Cl2 ——————> 2NaCl. One of the leftover
hydroxyl groups rip of a hydrogen from the isomerized safrole to form water, and the other hydroxyl group rips off a hydrogen from the piperidine,
forcing the nitrogen on the piperidine to bond with the carbon which has also had a hydrogen ripped off, forming a stable between the two. Another way
to put it is this: I start out with 2 moles of safrole, 2 moles of piperidine, 4 moles of NaOH, and 1 mole of Cl2 gas, and I end up with 2 moles of
NaCl, 2 moles of my piperidine safrole dirivitive, and 2 moles of water.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
1: None of those structures are a safrole molecule.
2: The disappearing carbon is the carbon on the precursor molecule you apparently think is safrole but isn't.
The carbon atom on that molecule which makes it not safrole is the one which disappears in the last reaction without explanation. You should be able
to see this if you understand the structures you have written.
3: What exactly does , "This reaction scheme was made in Sigma Aldrich" mean?
I'm fairly sure Sigma Aldrich knows what the structure of a safrole molecule is. Whoever wrote this clearly doesn't.
4: That reaction mechanism you described above sounds about as plausible as faith healing.
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Just for my own completeness, I will look at this. Not really my current interest, but it did connect.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
If one could animate safrole with just an amine cl2 and NaOH why would ppl go to the trouble of isomerizing and making the ketone then using mercury
or nabh4 to get to the same point.if this is something that works on paper just because the equation is balanced or whatever doesn't mean it's
possible in real life.is this even something that someone anywhere in the world has said works bcoz it just seems wrong.all of it doesn't make sense
and I'm just guessing here but adding safrole to NaOH and cl2 or what will equal NaOCl won't lead to the clorosafrole compound that when reacted with
piperidine would equal your final molecule and clorine won't be displaced by the amine unless it's turned into a bromine or iodine as cl is a poor
leaving group.forming the halosafrole would require acidic conditions not basic.and NaOCl with safrole just seems like a way to fuck up the safrole as
it might oxidize the double bonds or cleave it to form the aldehyde like kmno4 does.or it might just form tar.is there some complex formed I don't
know about by reacting the amine and the caustic clorine solution? I don't think any of this will work.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Looking at the picture provided the first step looks like the "safrole" is refluxed with piperidine and somehow the double bond dehydrates the
piperidine leaving an alkane and a partially dehydrated piperidine. This is probably wrong bcoz alkenes don't dehydrate alkylamine leaving the alkene
as an alkane.that doesn't happen does it.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
The second step is an oxidation that somehow reduces an alkane and partially dehydrated piperidine to an amine which is all wrong.none of this is
right.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Okay yeah I can see where I fucked up on this procedure. A lot of the atoms and bonds are missing or not where they are supposed to be, and I clearly
did not think this through as thouroughly as thought I did. Obviously, constructing Anaoulogs of compounds is clearly not MY specialty. I think an
animation of bromosafrole with piperidine sounds a bit more plausible than my procedure. Thanks for the suggestions, they help me out a lot, so ill
leave it at that.
|
|
|