Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
The colour of iron(III)acetate solution in dilute acetic acid
A small amount of iron(III)oxide/hydroxide was suspended in dilute acetic acid (~15% by volume). The suspension was orange-brown. Initially, the
oxide/hydroxide did not dissolve, but after boiling for 10 minutes a clear, light yellow solution was obtained.

I was surprised about how light the colour had become. I checked the CRC handbook, where I found that only basic iron(III)acetate has an entry in
chapter 4. So I assume that non-basic iron(III)acetate does not exist as a separable compound. From the Wiki I learned that iron(III)acetate occurs as
a special molecule containing 1 oxygen atom per 3 iron(III)acetate molecules:
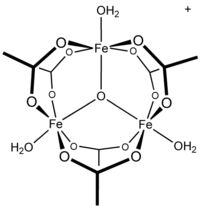
The colour of this compound is said to be brownish red (both Wiki and CRC handbook), and soluble in acids, but not in water (CRC Handbook).
So, my question is whether in dilute acetic acid, we just have [Fe(H2O)6]3+ instead of [Fe3(μ3-O)(OAc)6(H2O)3]+. Does anyone have any experience with
this?
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
[Fe(H2O)6](3+) is nearly colorless. A solution of this is very pale violet, but in concentrations of 1M or so, you cannot see this color. Salts, which
have only iron(III), coordinated to water ligands are pale grey/violet. Ferric ammonium sulfate, when really pure, indeed has such a color.
A yellow color usually is given to iron(III) by chloride ligands. A nice experiment is dissolving ferric nitrate or sulfate in dilute HNO3. This gives
a colorless solution. If you add a drop of solution of NaCl (or HCl), then the liquid turns yellow.
In strong acetic acid (15% or so is quite strong), I expect that there hardly is any coordination to acetate ion. Nearly all acetate will be present
as acetic acid, because this is a weak acid.I expect that if you increase the pH with some buffering agent (adding NH3 probably will do the job) then
the color will intensify, due to formation of more acetate ions.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Lab light is a source of UV. Acetate anion can be light sensitive (see https://books.google.com/books?id=JbZFAAAAYAAJ&pg=PA687&... ). The cited formation of CO2 may lead to a bicarbonate/carbonate salt.
Also, if one also assumes the release of an electron with UV light in the presence of ferric:
Fe(lll) + e- = Fe(ll)
We get some ferrous, or, even more likely, you may be starting with a ferric/ferrous acetate mix. Apparently, Fe(ll) can react with O2 (from air) and
H+, in an electrochemical reaction producing a basic salt:
Fe(ll) + 1/2 O2 + H+ --> Fe(lll) + OH-
Bottom line, the reaction may be more complex than expected! Try repeating absent lab light and a loose glass lid limiting oxygen exposure, and see
if you get the same results.
[Edited on 25-10-2019 by AJKOER]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Thanks for your replies. I was away this weekend and left the solution to evaporate in a dessicator over NaOH. Yellow crystals have formed.
My conclusion is that my starting material (iron oxide/hydroxide) was not pure. Indeed it came from a solution which contained chloride.
I do not believe that UV from the lighting will reduce iron(III) to iron(II) in this environment. But the test will show.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I expect that the effect, mentioned by AJKOER is only marginal. The amount of UV-radiation in the artificial light source, commonly used, is very low.
Even in bright sunlight I do not expect more than a marginal effect, unless the material is exposed for many hours or even days.
AJKOER, in nearly all of your posts I see a tendency to make things much more complicated than necessary. The effects you mention certainly may exist,
but only at trace levels, and in practice no one in an amateur setting without expensive special equipment will observe them. This answer again is a
typical example of your type of posts.
|
|
|