Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
Glycine from OTC materials
I stumbled on the idea of making copper(II) glycinate as a vibrant blue pigment, which I assume would just require dissolving glycine in a
Cu+2 solution. The problem then becomes getting my hands on the glycine. I mean, sure, I could just buy it, but that's no fun. Apparently
it could be made by amination of chloroacetic acid with ammonia, but as chloroacetic acid obviously isn't OTC either I'd have to make it, which
apparently would require chlorination (presumably with Cl2, not HCl) of acetic acid with acetic anhydride catalyst, so then I'd have to
make some acetic anhydride... and it just spirals far afield from what I'm trying to do.
Is anyone aware of an easier path to glycine via OTC materials, either by extraction from protein-rich food (seeds or pork rinds?) or synthesis?
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
The chlorination of acetic acid photocatalytically works on paper but the mixed chloroacetic acids produced are nearly impossible to separate on the
lab scale.
Your best bet for monochloroacetic acid is the hydrolysis of 1,1,2-trichloroethtlene using sulfuric acid where it can be obtained in high yield. The
trichloroethylene is purchasable fairly cheaply on eBay and can also be found in a number of consumer products. This method was used industrially so
there are quite a few references out there for high yielding procedures.
After that, the glycine synth is basically trivial. Simply letting a solution of monochloroacetic acid in ammonia sit around for a while does the
trick with a little workup. Orgsyn has the procedure.
|
|
|
Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
I thought the canned air at my local hardware store was trichloroethylene, but it turns out to be dichloroethane. Which is a smidge different.
Do you think glycine could be synthesized by reductive amination of the aldehyde group on glyoxylic acid in the presence of ammonia? It sounds like
glyoxylic acid could be made by reduction of oxalic acid, which I have on hand, although all the procedures I'm found for making glyoxylic acid from
oxalic acid are electrosyntheses, which I have no experience with. But if I could make glyoxylic acid, would that even be a viable way of making
glycine? Would it require a reducing agent, and if so, are there any OTC ones (i.e. not NaBH4) that would have a good shot at working? (Maybe hydrogen
gas? Could bubble it into solution from an HCl/Zn hydrogen gas generator)
|
|
|
Arcaeca
Hazard to Self
 
Posts: 70
Registered: 24-8-2019
Location: Kansas, USA
Member Is Offline
Mood: brøthér, can you spare some B̲̺̹̙̑́̓́ͧ̎ͭ̈́͜L̰̦̼̻͈͖̺͔̇̇̿ͪ̓̃̽ͦŲ̘̲̻͔̀͌͑͑̊͛̑̀͊̕E̐ͮͯ͆̔̾͘͏҉̥̫
|
|
Okay, I've done some more snooping and glycine via reductive amination of glyoxylic acid is a thing. Then the problem is getting my hands on
glyoxylic acid.
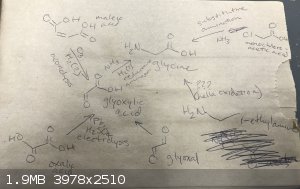
These are all the paths to glycine I know about :
Maleic acid --> ozonolysis to glyoxylic acid --> reductive amination to glycine. Problem: requires an ozone generator, which I don't
have, and generates ozone in quantities that are dangerous to work with
Oxalic acid --> electrochemical reduction to glyoxylic acid --> reductive amination to glycine. Problem: reportedly low and impure yields,
have no experience with electrolysis, have no electrical source on hand and would have to go get a car battery or something, best H2SO4 I have to work
with is 93% drain cleaner w/ a metal inhibitor and idk how well that would work, or if 31% HCl could work instead
Glyoxal --> oxidation to glyoxylic acid --> reductive amination to glycine. Problem: both formation of glyoxal and the oxidation step
typically use nitric acid as the oxidizing agent, which I don't have easy access to
monochloroacetic acid --> substitutive amination to glycine. Problem: production of monochloroacetic acid is typically done with chlorination
of acetic acid with Cl2, which I don't have the equipment to safely generate and bubble into solution, or else oxidation of trichloroethylene, which I
don't know where to get
ethylamine --> oxidation to glycine. Problem: I have literally no idea how well this would work, I just thought of it, oxidation of the
alkane part all the way up to a carboxylic acid would probably have a ton of ketone/ester/alcohol side products, would probably require an oxidizing
agent poweful enough to turn the amine group into an amide group or something
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Gelatin can be hydrolyzed to give amino acids, and is 22% glycine. Separating it from the other amino acids would be a challenge, though (I found a
paper that described how it can be removed from mixed amino acids by precipitating it with copper picrate, but that seems a hell of a reagent to work
with).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
what about oxidation of Ethanolamine?
"A mind is a terrible thing to lose"-Meisner
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
There are a number of problems with the aforementioned methods. Most industrially used methods do not work on the lab scale and are typically carried
out under pressure, at high temperature, and over specially designed catalysts. The yields are often low, but the plant employs recycling and
separation techniques to facilitate a continuous flow. The ozonalysis, for instance, also involves a hydrogenation step over a catalyst, if you read
the relevant literature.
Some of these will also be difficult and impractical due to issues separating glyoxal, glyoxylic acid, and oxalic acid which tend to have many
hydrates, hemiacetal dimer hydrates, etc. This mixture exists as a syrup which is a real pain to dry and/or separate anything useful from. I have
attempted glyoxal synthesis via air oxidation of ethylene glycol before, which was evidently successful however the workup proved to make the process
impractical over the standard route using selenous acid and paraldehyde.
I still highly recommend the chloroacetic acid method. Refluxing trichloroethylene with 75% sulfuric acid solution will do the trick, with the
byproduct being HCl that can be collected. Separation of the monochloroacetic acid is trivial with distillation or acid/base workup. Letting the
monochloroacetic acid sit with aqueous ammonia gives glycine in about 70% yield which is crashed out with methanol and recrystallized from the same.
(Orgsyn)
You can find trichloroethylene in products like this which come in quart, gallon, or spray can form.
|
|
|