Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
Oxidising uric acid?
I came across uric acid while on wikipedia,( https://en.m.wikipedia.org/wiki/Uric_acid )
and I thought that maybe, it could be modified to be energetic to some extent.
If those are secondary amines (forgive me, but organic chemistry is not my field)
Could they be oxidised to somehow?
Maybe even hydrogenated first, then nitrated?
Any thoughts about it?
EDIT: removed a text based illustration of uric acid that didn't show properly.
[Edited on 30-10-2019 by Kobold vor NH4]
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Most likely the N-H bond could be nitrated. Now i have no idea how stable it would be. Also those C=O bonds are not that much energetic.
[Edited on 30-10-2019 by underground]
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
Quote: Originally posted by underground  | Most likely the N-H bond could be nitrated. Now i have no idea how stable it would be. Also those C=O bonds are not that much energetic.
[Edited on 30-10-2019 by underground] |
So what would the N-H look like after nitration?
With the carbonyl, that's why I was thinking to hydrogenate it, then nitrate it.
Of course, I'm just exploring the possibility of it, because I don't have any nitric acid at the moment.
I put together this drawing, because it's easier to visualise.
The green is for the amines.
The red is for the nitrated carbonyl.
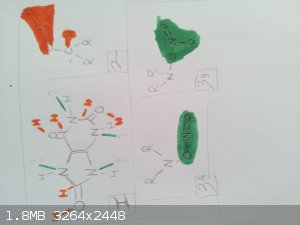
"I don't need no excuse for being what I am"
-----Frank Zappa
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
In theory, it should look like this
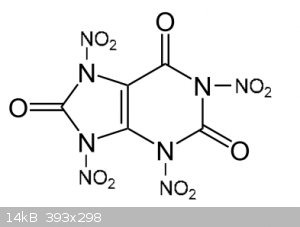
[Edited on 31-10-2019 by underground]
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
Right, so it's turning the secondary anime into a nitroamine, correct?
So the combustion formula should be,
[2 C2(CO1)3(NNO2)4 --->> 10 CO2 + 8 N2 + O2]
Is that right? So it looks it's got plenty of oxygen with that nitration, but would it be stable?
Once i get some more nitric acid, I'll probably try making it on a 1~5g scale (with all of the appropriate safety gear on of course!)
EDIT: I typed nitroamine, but autocorrect changed it to nitrosamine. How embarrassing.
[Edited on 1-11-2019 by Kobold vor NH4]
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Not a nitrosamine but a nitroamine (N-NO2). Nitrosamines have formula N-NO
I don't know of any good ways to get all those nitro groups on there, though.
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Metacelsus  | Not a nitrosamine but a nitroamine (N-NO2). Nitrosamines have formula N-NO
I don't know of any good ways to get all those nitro groups on there, though. |
Even WFNA most likely won't be enough, some acetic anhydride may be required.
Why you actually wanna try something like this, there are plenty of other better options out there.
[Edited on 1-11-2019 by underground]
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
I wonder, would concentrated H2O2 work?
Besides, im just exploring the possibilities of different energetics.
|
|
|
underground
National Hazard
   
Posts: 692
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
H2O2 unfortunately will not work
|
|
|