numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Synthesis of Boron subphthalocyanine chloride (with pictures!)
Related to the aza-subphthalocyanine I made a few weeks back but this time making the parent unsubstituted version.
Just as a fun fact, the "sub" phthalocyanine refers to the fact that this species incorporates 3 dinitrile units, whereas a more common metal
containing version usually contains 4.
This dye is commercially available and likely more well known, and with the easy synthesis1 I gave it a go.
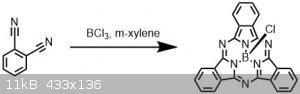
1,2-dicyanobenzene (512 mg, 4 mmol, 1 eq) was added to a 20 ml vial with a septum cap and cycled with N2. Dry m-xylene (4 ml) was added,
then BCl3 (1 M in DCM, 4 ml, 4 mmol, 1 eq) was added dropwise with stirring. The reaction was heated to 120 °C and a purge needle added.
The DCM was boiled off under N2, after which the purge needle was removed and the reaction heated for another hour.
Over the course of the reaction, the color changed from colorless to yellow, to red, to black.
 
After an hour I let the reaction cool after which it looked very colorful.

I used DCM to transfer the thick mixture to a flask and then removed the solvent under reduced pressure.

I had difficulty removing all the solvent so I decided to just crash it out with some methanol and filter over celite.

My hopes where to just extract it off afterwards. Unfourtunately the stuff has horrible kinetic solubility and just wouldn't redissolve. each time I
ran 20 mL DCM through it I got around a mg out. Nice color here but that's probably only a few mg.

So I had to resort to a soxhlet extraction. Should have filtered the solid directly, and not on celite...

Once the celite ran colorless, I had a homogenous solution in the collecting flask and concentrated it down into a 20 ml vial on the rotovap. Ended up
getting 98 mg (17%, vs 82% lit yield) of a black, slightly purple/green solid. Few comments about the yield: p-xylene is generally used, I didn't have
any so used m-xylene instead. The prep heated to relux, I heated to 120 °C, and the mechanical losses from all the filtering and recovery attempts I
did likely resulted in a loss as well. So correctly done, 80% might be doable.

This dye is bright pink in solution and is slightly fluorescent. The emission is orange, but the pink kind of drowns it out, even under high
intensity 365 nm it's barely visible by eye.
 
1. European journal of organic chemistry, 2003, pp 2547
[Edited on 11-11-2019 by numos]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Nice work numos and I love those colours!
A couple of question I have, 1) why is BCl3 used in equimolar amounts to the dicyanobenzene (apart from the fact that the procedure calls for it!)?
2) could you use BF3 instead? I have the latter in 2 different solvents but BCl3 is harder to acquire. I suppose you would end up with a fluoride
equivalent.
|
|
|
Texium
|
Thread Moved
11-11-2019 at 08:47 |
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Looks lovely! Nice to see a fresh synthesis write-up on here.
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Boffis  | Nice work numos and I love those colours!
A couple of question I have, 1) why is BCl3 used in equimolar amounts to the dicyanobenzene (apart from the fact that the procedure calls for it!)?
2) could you use BF3 instead? I have the latter in 2 different solvents but BCl3 is harder to acquire. I suppose you would end up with a fluoride
equivalent. |
1.
The excess of BCl3 over the dinitrile may have two reasons:
BCl3 is quite volatile, while I was removing DCM copious amounts of smoke came out as well, I imagine excess BCl3 helps with this.
The other fact may simply be that BCl3 is much cheaper on a per mole basis than the dinitrile. Using the expensive reagent as the limiting one makes
sense. As for stoichiometry concerns... I couldn't tell you, they get >80% yield which I guess means by-products are minimal.
2.
The fluoride analog does exist, albeit it's much less studied. Based on what I found, it can only be made from the corresponding chloride, either
through AgBF4 or by first substituting the chloride with phenol and then treating with BF3.... Sorry dude, can't make these without BCl3, but stay
tuned - I am working on several different dyes that can be made using BF3 etherate!
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Good write up of a very interesting and very colorful compound.
The starting materials look like challenge from OTC materials.
I guess there must be a route to the phthalonitrile from phthalic acid and ammonia. Make the amine from the acid chloride then if I remember
correctly a copper salt to the nitrile. Ring formation could will probably be a major problem. There is probably a route from Salicylic acid too
Did you make the nitrile, if so how?
PS: According to wiki it can be synthesised by the dehydration of phthalimide by boiling in acetic anhydride. It does give a reference but its in
German. Presumable the yield must be less than 50%
The BCl3 from the oxide +carbon and chlorine at 500C. The chloride has a 12C boiling point so I would prefer the bromide as it boils at 92C and can be
made the same way.
[Edited on 11/13/2019 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by wg48temp9  |
Did you make the nitrile, if so how?
PS: According to wiki it can be synthesised by the dehydration of phthalimide by boiling in acetic anhydride. It does give a reference but its in
German. Presumable the yield must be less than 50%
The BCl3 from the oxide +carbon and chlorine at 500C. The chloride has a 12C boiling point so I would prefer the bromide as it boils at 92C and can be
made the same way.
[Edited on 11/13/2019 by wg48temp9] |
I agree that the starting materials can be a challenge for someone starting out. I did not make this sample of phthalonitrile, as I was fortunate to
scoop it from a junkyard.
However it seems that it can be made easily from o-xylene, AlCl3, t-Bu nitrite (can be made from tBuOH), and hydroxyphthalimide (can be made from
phthalic anhydride and hydroxylamine in 86% yield). I didn't study the ref, but it seems like 70% yield, not too bad:
Journal of the American Chemical Society, 2016, 138 (10), 3294-3297
BCl3 sounds terrible to make, it's a nasty gas and also incredibly cheap as a DCM solution, so hardly seems worth it to risk to make. I thing BBr3 is
also horrible, even with a schlenk line - screws up your expensive rubber tubes 
|
|
|