vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
Any method to convert potassium dichromate to ammonium dichromate?
I have got almost 200g potassium dichromate. Is there any way to convert it into ammonium dichromate?
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Convert it in to potassium chromate with KOH and then make ammonium chromate by fractional crystallization (ammonium chromate have much lower
solubility than potassium chromate). After separation of ammonium chromate just make its acidified solution and let evaporate most of water.
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
How did you make ammonium chromate
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
add ammonia or maybe an ammonium salt
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
Adding ammonia to potassium chromate can't displace the chromate ion to ammonia . Can it?
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
The problem is ammonium chromate solution is rather unstable and evaporating it down may be difficult without partial reduction of the Cr6+ and also
loss of ammonia.
I used to make ammonium dichromate from either strontium or barium yellow. These pigments where once easily obtained and cheap but now seem to have
disappeared from the market, they are strontium and barium chromate respectively. I would treat say strontium chromate with just enough dilute (2M)
sulphuric acid to convert it into strontium dichromate which is soluble and strontium sulphate. Without separation of the ppt I would then precipitate
the remaining strontium with ammonium sulphate from garden fertilizer, stand in the dark for a couple of days, decant and filtering the ammonium
dichromate solution. This is then evaporated down slowly on a water bath in subdued light but a good draft. Bright sunlight and too high a temperature
give a brown product. More can be recovered by washing the strontium sulphate ppt with water. You can filter off the strontium sulphate but it filters
slowly. The strontium may be recovered from the sulphate precipitate if required.
Since you have potassium dichromate you would have to convert it into strontium or barium chromate first. To do this make up a strong solution,
neutralise with NaOH or KOH solution and add an appropriate alkaline earth chloride. Since you are making it as an intermediate you can also use
calcium chloride or nitrate and precipitate calcium chromate but in this case you may find the final ammonium salt to be slightly contaminated with
gypsum.
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
I've got barium chloride in excess. So I first make barium chromate then I add a small amount of Sulfuric acid just to make barium dichromate? But
that would make barium sulfate precipitate out right? Can you clarify the steps from that point onwards?
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
One way to make ammonium dichromate is adding a solution of ammonium perchlorate. KClO4 then precipitates from the solution and ammonium dichromate
remains behind.
The KClO4 then can easily be purified and made snow white and the ammonium dichromate can easily be made to crystallize by allowing the solution to
evaporate.
If you don't have ammonium perchlorate, then it will be very hard to make ammonium dichromate from the potassium salt. The potassium salt is less
soluble than the ammonium salt.
But, if you have this material from a seller, then why not contact that seller and ask him to send you ammonium dichromate?
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by vibbzlab  | | I've got barium chloride in excess. So I first make barium chromate then I add a small amount of Sulfuric acid just to make barium dichromate? But
that would make barium sulfate precipitate out right? Can you clarify the steps from that point onwards? |
Yes, you get an orange solution of barium dichromate and much barium sulphate ppt but you don't need to remove it at this point because the dichromate
solution is a bugger to filter, too fine for glass sinters and too corrosive for filter paper.
The equation is roughly:
2BaCrO4 + H2SO4 -> BaCr2O7 + BaSO4 + H2O
Heating almost to boiling and cooling slowly helps make the barium sulphate precipitate more readily filterable or settle faster.
You then add half a molar equivalence of ammonium sulphate solution:
BaCr2O7 + (NH4)2SO4 -> BaSO4 +
(NH4)2Cr2O7
Allow the bulk of the barium sulphate to settle and then pour off the clear solution with the minimum amount of ppt and then vacuum filter. You can
recover a little more ammonium dichromate by washing the ppt again with fresh water
Evaporate slowly and in a good draft, I used a shallow ceramic bowl on a pan of boiling water. Some guys use hair driers to create a draft but I have
found that this is not necessary particularly if you do it outside.
If I were starting from potassium dichromate I would make up a fairly strong but cold solution of this salt then neutralise with sodium hydroxide
solution, heat to boiling and add the barium chloride as a 1M solution (also hot) and cool slowly to room temperature. The resulting precipitate of
barium chromate will be fine and filter slowly so just let it settle for a few days, pour off the salt solution and replace with clean water. Stirr up
and let settle again to wash the ppt and then use this ppt for the next stage without isolation or drying of the barium chromate which has the form of
a brilliant lemon yellow powder.
You will need to calculate the quantities carefully, the reactions are practically stoichiometric.
Hope this helps.
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
Thank you so much . This really is helpful I will try this soon
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Somewhere, in one of my very old note books I have a detailed "as done" write-up but I can't find it at present.
Incidentally I have found that one of my old suppliers still sell pure barium yellow. If any one is interested U2U me for the address.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Following Boffis idea, but a bit more simple and gentle (no heating involved), try just adding aqueous (NH4)2SO4 to the potassium dichromate. Stir to
dissolve and attempt to freeze out the potassium sulfate hydrate.
I have done this successfully when the starting potassium salt was KNO3.
My source of (NH4)2SO4 was created by the action of NH3 on commonly available (and pure) Epsom Salt.
[Edited on 8-12-2019 by AJKOER]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@AJKOER, While I haven't tried this simple direct method on these reactions I did recently try this to prepare pure KClO3 from weedkiller made of 1:1
NaCl:NaClO3 by adding KCl as I reported elsewhere on SM. It was completely successful but it took a lot of time and numerous recrystallisation to get
rid of the sodium and chloride from the potassium chlorate. It is much easier if you can remove one set of ions as an insoluble compound. Your method
sounds easy but would require a lot of recrystallising and heating, believe me. This is if it works at all. You may find that ammonium chloride is
better, forcing the ammonium dichromate out of solution leaving the KCl in solution, I am guessing here but not without a bit of experience.
One final point is that I developed the technique above because of the availability of strontium and barium chromates as pigments and there is an
interesting point about the sequence of chemical reaction. You may wonder why not just mix the alkaline earth chromate with ammonium sulphate and
acidify with sulphuric acid? Answer: because the yield drops from 87-88% to about 60-62%. I am not sure why but this is what I found. There seems to
be a lot of chromate left in the barium sulphate ppt. You can only know this from experience gained from actually doing such experiments and the
Devil, as they say, is in the detail! This is why I post some of the reports I do like the KClO3 from old weedkiller, the theory is easy the detailed
procedure more difficult.
By all means have a go and keep us informed with your quantitative data... please  . .
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
That's odd, calcium chlorate/chloride seems to work OK for that. But anyways if you're going to remove ions well remove them and make CrO3. The
sulfate or bisulfate or whatever precipitate can be fairly clean and clumpy so I can't imagine anything ultimately giving a higher yield.
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Since this topic has been brought up, i had an idea a while back.
If we were to deprotonate potassium dichromate with ammonium carbonate to form ammonium potassium chromate, (NH4)KCrO4
then protonate the solution with H2SO4, would we then get ammonium dichromate since from what i can find the PKa of ammonium dichromate is 11.34 and
the PKa of potassium dichromate is -2.3, meaning the more acidic might be less favorable.
K2CrO7 + (NH4)2CO3 -----> (NH4)KCrO4 + CO2
(NH4)KCrO4 + H2SO4 -----> (NH4)CrO7 + KHSO4
I realize potassium dichromate is still much less soluble than potassium bisulfate which may prevent us from evaporating the solution down to
precipitate ammonium dichromate, but this may atleast prepare a solution of ammonium dichromate?
As an alternative perhaps a less acidic acid could be used, one thats weaker than potassium dichromate but stronger than ammonium dichromate like
phosphoric acid.
Ammonium dichromate PKa: http://www.t3db.ca/toxins/T3D0700
Potassium dichromate PKa: https://www.drugbank.ca/salts/DBSALT002757
No doubt there are better methods already suggested but im still curious if this would work.
Sufficiently advanced science is indistinguishable from madness.
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by woelen  | One way to make ammonium dichromate is adding a solution of ammonium perchlorate. KClO4 then precipitates from the solution and ammonium dichromate
remains behind.
The KClO4 then can easily be purified and made snow white and the ammonium dichromate can easily be made to crystallize by allowing the solution to
evaporate.
If you don't have ammonium perchlorate, then it will be very hard to make ammonium dichromate from the potassium salt. The potassium salt is less
soluble than the ammonium salt.
But, if you have this material from a seller, then why not contact that seller and ask him to send you ammonium dichromate? |
Ammonium perchlorate is available on ebay uk . It a bit expensive at £9 for 200g
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Fairly expensive, but at least a perchlorate remains available for home chemists in the EU. The K-salt and Na-salt are forbidden (they are much
cheaper and can be abused for making explosives, much better so than the ammonium salt). The K-salt imho is not very useful for general home
chemistry, it is nearly insoluble in cold water. It only is useful for pyrotechnics. The ammonium salt is a little more interesting, e.g. for making
other ammonium salts from potassium salts.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Can't you just convert it to chromium trioxide using sulphuric acid? From there it should be trivial to make ammonium dichromate.
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
I was wondering how efficent the potassium dichromate and ammonium perchorate method would be.
From my calculation if you mix stockometric ratios of saturated solution of the reactants at 100C then cool the solution down to 25C, about 96% of
the perchlorate will crystallise out of solution leaving you with a saturated solution of ammonium dichromate contaminated by only a few percent of
perchlorate, probably fine for a volcano or copper chromite prep.
All I need to do now is figure out was the water volumes should be. But without density info on the solutions the only way i can do that is assume
the volume of the reactant don't change when dissolved which I understand is inaccurate. I guess I will have to measure them.
Below is my work sheet. Comments welcome. The data is from wiki.
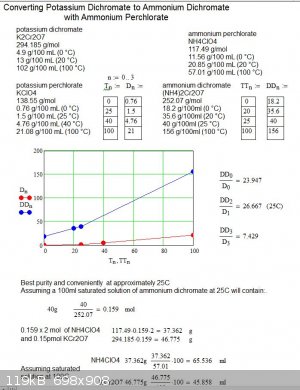
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Ammonium bitartrate may work just as well as ammonium perchlorate.
There is a potential difficulty in that dichromate can potentially oxidize bitartrate, but performing the reaction at suitably low temperatures should
suppress this. Potassium bitartrate is even less soluble than the perchlorate.
EDIT: Ammonium persulfate should work reasonably well also, it is used as PCB etchant IIRC.
EDIT EDIT: Combining perchlorate and dichromate sol'ns at 100 C sounds concerning to me.
[Edited on 19-12-2019 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Combining perchlorate and dichromate is not a problem at all. I even heated such mixes well above 100 C, no reaction at all. Both are oxidizers and
both are quite stable.
I have done the reaction at temperatures of 80 C or so (not completely at boiling temperature, because I then had premature formation of crystals of
indeterminate composition). I dissolved NH4ClO4 in water at 80 C, almost saturated. The same for K2Cr2O7. Then I heated both solutions to 100 C and
mixed them. On slowly cooling down you get crystals of KClO4, which can be separated easily. The remaining solution is mainly (NH4)2Cr2O7.
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | Ammonium bitartrate may work just as well as ammonium perchlorate.
There is a potential difficulty in that dichromate can potentially oxidize bitartrate, but performing the reaction at suitably low temperatures should
suppress this. Potassium bitartrate is even less soluble than the perchlorate.
EDIT: Ammonium persulfate should work reasonably well also, it is used as PCB etchant IIRC.
EDIT EDIT: Combining perchlorate and dichromate sol'ns at 100 C sounds concerning to me.
[Edited on 19-12-2019 by clearly_not_atara] |
Thanks for reminding my why pot bitartrate crystallises out of wine.
Below is a better a graph showing the solubility of all the salts in a valency x mol basis. The reactants must be along the same horizontal line to
be stockeometric. The reactants must be grater than the low soluble potassium salt line in order to be deposited and below the ammonium dichromate
line so that the dichromate will remain in solution.
Notice on a valency.mol basis the limitation of an RT reaction is the low solubility of potassium dichromate. That can be improved by using a hot
potassium chromate solution.
Also the solubility of potassium bitartrate is much lower than the perchlorate and suggests a RT reaction is possible with resonable yeild of the
ammonium dichromate assuming the tartrate is not significantly oxidised.
Chromates have better solubility profiles but then the product would have to be converted back to the bichromate.
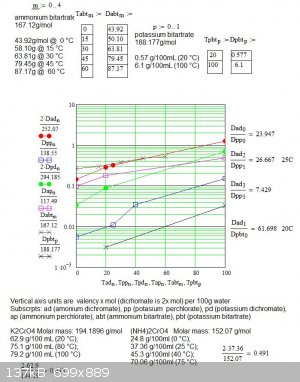
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by woelen  | Combining perchlorate and dichromate is not a problem at all. I even heated such mixes well above 100 C, no reaction at all. Both are oxidizers and
both are quite stable.
I have done the reaction at temperatures of 80 C or so (not completely at boiling temperature, because I then had premature formation of crystals of
indeterminate composition). I dissolved NH4ClO4 in water at 80 C, almost saturated. The same for K2Cr2O7. Then I heated both solutions to 100 C and
mixed them. On slowly cooling down you get crystals of KClO4, which can be separated easily. The remaining solution is mainly (NH4)2Cr2O7.
|
Thanks that confirms the theory. I would expect your remaining solution to be greater than 90% pure. I don't have any tartaric acid so it will be the
perchlorate method for me. Though I have ordered some as it could be useful for converting a different potassium salt.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The crystals of KClO4 are a nice byproduct. You can put those crystals on a piece of filter paper (or coffee filter) and put that on a pile of paper
tissue, so that most liquid is sucked away from this. You then get a yellow crystal mass. Next, you can dissolve that in as little as possible of
near-boiling water and allow to recrystallize again. If you cool down to just below zero degrees, then you recover nearly all KClO4. A second
recrystallization makes your KClO4 white as snow. If you just want to use the KClO4 for pyrotechnic purposes, then a single recrystallization already
is sufficient, but the product then is not purely white.
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
So using ammonium perchlorate to convert potassium dichromate to the ammonium salt means its difficult to purify the ammonium dichromate from the pot
perchlorate because the perchlorate is less soluble on a valency x mol basis.
However ammonium chloride can be used because potassium chloride is significantly more soluble than ammonium dichromate on a valency x mol basis so
the ammonium dichromate can be purified by simple crystallisation.
Below is a copy of the previous graph I posted with the addition of the solubility curves for potassium chloride (top curve), and ammonium chloride
(2nd from top). The 3rd from the top curve is the ammonium dichromate curve which is less than 1/10 as soluble as the potassium chloride on valency x
mol basis.
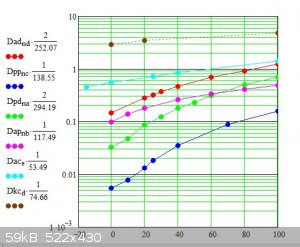
A a similar process can be use to convert potassium permangante to ammonium permanganate. NOT RECOMMENDED for volcanoes
Preparation of ammonium permanganate from prepchem.com
"A solution of 16 g of potassium permanganate and 44 g of ammonium chloride in 300 ml of water at 70° C is filtered through asbestos to remove traces
of manganese(IV) oxide and evaporated on the steam bath to a volume of 200 ml. The hot solution is re-filtered as before and cooled to 5° C in an ice
bath. The felted needles of ammonium permanganate are drained thoroughly in a sintered-glass suction funnel and dried in vacuo. The yield of ammonium
permanganate is 11 g. The crude ammonium permanganate may be recrystallized from water at 60-70° C.
Inorganic laboratory preparations, by G. G. Schlessinger, 39, 1962"
[Edited on 12/22/2019 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|