Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
From 'Conductive silver liquid' to silver iodide & dichromate
I was given a small bottle of very old "conductive silver liquid". See photo. Looking up the suppliers specs and sds showed that originally it was
made from approx. 56% very fine silver and the rest mainly Toluene and Butanone with various other stuff. It was completely dried up when I got it, it
was just a hard ball of light brown - greyish color.
I was keen to extract the silver. I cut open the bottle and removed the dried up contents. This I then soaked in Toluene overnight. The next morning
it was soft and I could break it up. I had no more toluene so added xylene and agitated with the stir bar for a few more hours. I filtered the mixture
and washed more xylene through a few times which left me with a light brown powder with some silver specs. This I left overnight to dry. Afterwards it
was weighed at 15g.
I reacted this powder with 25ml 70% nitric acid. The reaction was initially quite fast with lots of nitrous oxides being released. See photo. When it
slowed down I switched on the stirring to keep it mixed well. After 2 hours I was left with a large volume of white powder. I decanted off as much
fluid as I could. I then dissolved a tiny amount of the white ppt in water and added potation iodide solution; a yellow ppt was obtained suggesting
the white powder indeed container silver nitrate.
I then heated up the white powder to drive off any remaining acid. Initially some more nitrous oxide was expelled but then it seemed to melt into a
light golden fluid. When I cooled this down it formed glassy strands which became like plastic upon further cooling. This stuff was not soluble in
water. See photo. What can this be? A reaction between the nitric acid and some of the solvents that was in the original item?
I then boiled the lot with excess water for 30 minutes in an attempt to extract the silver nitrate that should have been present. I divided the
resulting solution in 3 parts.
The first part was mixed with a solution of 9g potassium iodide in water. The result was a yellow ppt, should be silver iodide AgI. This was washed by
decanting with cold water a few times. The yellow ppt was then dried and weighed at 6.9g. Although I am not sure it was fully dried. See photo. This
was 2 days ago, and the yellow color has by now slowly changed to light brown as it is in a clear glass bottle so not protected from light. As
expected.
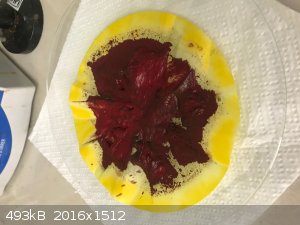
The second part of the silver nitrate (most likely) solution was mixed with 7g of potassium dichromate. I gave a dark red ppt with the excess
potassium dichromate solution on top. See photo. That was filtered, and the residue washed by agitation with cold water until the filtrate changed
from light orange to very pale yellow. See photo of the washed residue. This was then dried overnight in air. See photo of the semi dried dark red
powder in the crucible. The crucible in now in a desiccator under vacuum and with sodium hydroxide to get it fully dry.
I still have one part of the silver solution left. Any suggestions what I can do with this to get a interesting colored salt? I was thinking of silver
permanganate, but seems this can be explosively unstable under certain conditions?
|
|
|
Sulaiman
International Hazard
    
Posts: 3558
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
This stood out to me;
Quote: Originally posted by Lion850  | ... the suppliers specs and sds showed that originally it was made from approx. 56% very fine silver and the rest mainly Toluene and Butanone with
various other stuff. It was completely dried up when I got it, it was just a hard ball of light brown - greyish color.
... Afterwards it was weighed at 15g.
I reacted this powder with 25ml 70% nitric acid. ... I decanted off as much fluid as I could. ... |
Assuming that the toluene and butanone had mostly evaporated you should have had mostly silver as your starting point.
(Did you weigh it ?)
silver nitrate is extremely water soluble, 170g/100ml@10oC (10M)
there would have been enough water in your 70%HNO3 and produced by the reaction to dissolve most of the silver nitrate formed.
So I think that you decanted away most of your silver 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Sulaiman it was the small 25g bottle. SDS says silver content is 56%; I don't understand exactly if this % is by weight or volume if by weight the
silver would have been some 14g. Unfortunately I did not weight it before agitating with the solvents (should have).
What surprised me was that when the dried powder from after it was broken up with the xylene was reacted with 70Sulaiman% nitric acid there was a
voluminous ppt of a white powder. It seemed more then the original light brown (with silver specs) powder. This white powder easily dissolved in
water, and then gave a yellow ppt with potassium iodide.
So what was this white powder that seemed to be a water soluble silver compound but that did not resolve in the reaction acid? And then there is also
the glassy / plasticky material that appeared when the white powder was heated to boiling, and which is not soluble in water.
I can only guess that some other reaction was taking place between the acid and either the xylene or another solvent that was still left from the
original formula. The SDS lists some 10 or more other chemicals that can be present in small quantities.
So far I have the 6.9g of silver iodide, tomorrow I will weigh the dichromate which should be bone dry by then and then I still have the unused last
one third of the solution. I should be able to work out how much silver I reclaimed in the end.
|
|
|