Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Report on making Neodymium iodide
The members that followed my iodide posts will remember that my attempt to make NdI3 starting with the "neodymium" metal powder was a failure as it
seemed the powder was heavily contaminated. So I tried a different way, starting with my neodymium oxide Nd2O3, obtained as a pale blue powder from a
ceramics supplier.
- Add 6.5g H2SO4 (>95%) to 140ml water
- Add 7g N2O3 and stir. The suspended oxide quickly dissolved into a purple solution.
- Add 1.5g N2O3 to ensure the oxide is in excess. Some oxide powder remained in suspension. Filter to remove the excess oxide. Light purple solution
with blue oxide powder remaining on filter paper. Photo 1.
* 140ml water was used as Nd2(SO4)3 is not very soluble in hot warm water.
- Transfer to 1,000ml beaker and increase volume to 600ml (due to low solubility of lead iodide).
- Add 13g lead iodide PbI2 and stir. See photo of the yellow solution.
- Start heating with the hope that heating and cooling cycles with the opposing solubility behaviour of Nd2(SO4)3 and PbI2 will drive the double
displacement forward.
- After only 15 minutes the yellow was already fading. Sucked off a few ml of solution and tested against lead nitrate - the observed yellow ppt
showed that there was already a soluble iodide in the solution.
- After a few hours it seemed the solution was going more yellow again. Added a bit more Nd2(SO4)3 solution which I earlier prepared. I was not
concerned about having an excess of Nd2(SO4)3 as this should ppt out as reduced volume when boiling; but I did not want to have PbI2 left in solution.
- After 2 hours or so of stirring, boiled the solution down to 300ml and filter hot.
- The remainder was white (should be lead sulfate) with some purple (neodymium sulfate). Solution was purple.
- Continue boiling down and hot filtering twice more at 100ml and at 40ml. Remainder now only tiny bits of neodymium sulfate.
- Transfer last 20ml to crucible in sand bath. As the volume decreased the temperature increased the color went from purple to yellow-brown-ish.
- Removed from sand bath when a sticky liquid with crystals. It must have been dissolved in its own water of crystallization because as it cooled it
became completely dry and hard.
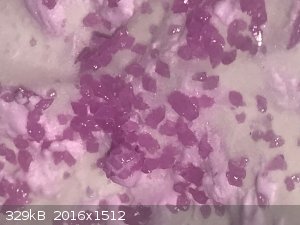
- Break up and transfer to vial, 9.6g of pale pink/lavender crystals. See photo.
Test of the product:
- Hygroscopic, gets wet in air.
- Dissolves very easy in small amount of water.
- Gives bright yellow ppt with lead nitrate.
Looking at the specification from some suppliers online, they say NdI3 is green. The only picture I could find of NdI3 is on the facebook page of
Poormans Chemist; he made his product from HI and Nd metal and his color is similar to mine, just slightly darker.
If anyone has experience with NdI3 or a reference that describes it please comment on the color I got.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Well, there is this which is a similar colour.
A quick google search has the fluoride, chloride, sulfate and nitrate a similar colour to what you obtained so I would say you are right on the money.
|
|
|
sciece nerd
Harmless

Posts: 25
Registered: 27-8-2019
Member Is Offline
|
|
A small typo:it's Nd2O3 instead of N2O3
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Interesting. I never got to preparing it myself, but indeed NdI3 is reported to be green. This counts for the water free compound, however, and I
think you may have a compound which is dehydrated but not water free.
If I remember well, NdCl3 is subject to hydrolysis when dried hot, so I imagine that NdI3 may also loose some HI and leave behind a (hydr)oxide.
I wonder whether it is necessary to start with a large volume of water. Double displacement reactions can run well if one of both compound is solid
and the product formed from it is also a solid. It is a way to convert PbSO4 to PbCO3 by treating it with Na2CO3 solution, for example.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
As mentioned above and also elsewhere the fact that my iodide is hydrated is most likely why it is not green.
Bezaleel - when I made Erbium iodide in a similar way I initially tried with a smaller volume of water as I assumed the lead iodide swirling around
will dissolve as it is consumed. It seemed to take very long though until I increased the water volume. However this is by no means conclusive as
there was too many other differences. With the Neodymium it may well have worked as quick as it did with a smaller volume.
I still want to try such a displacement using barium iodide which is much more soluble than lead iodide. But the lead iodide is easy to make.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The hydrated salt (the 9-hydrate) is pink. Very much like the pictures you made.
Here is a picture of an ampouled commercial display sample: https://chemcraft.su/product/21825
You did a good job in making this compound yourself!
[Edited on 14-2-20 by woelen]
|
|
|