sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Fuchsin confusion
Hi all,
From an eBay seller I bought a chemical labelled "Red Fuchsin Basic" (see attached photo). It was made by CENCO and has catalog number 39638.
I thought I was buying rosanaline hydrochloride (to perform the Schiff test for aldehydes) but now I am confused because some sources suggest that
"Basic Red Fuchsin" refers to a mixture of rosanaline hcl, pararosaniline, New fuchsine, and magenta II.
I haven't been able to find any online references to the original catalog number.
The product is dark green crystals which have an amazing ability to turn a lot of water pink for even a tiny amount of powder. I accidentally spilled
maybe 5mg and it took about a half hour of scrubbing with water and acetone to get the pink color off the sink and countertop.
Any idea how to tell what I have?
Thanks,
Sean

|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
I don't know if this is a clue which might allow someone to help me out, but I did some tests which showed that when this substance is dissolved in
water, the initial color is red if concentrated and pink if dilute. Further, lowering the pH to about 1 turns it a light yellow color (the pink
disappears). Raising the pH to about 13 makes it go colorless. Both of these changes are reversible.
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by sbreheny  | | I don't know if this is a clue which might allow someone to help me out, but I did some tests which showed that when this substance is dissolved in
water, the initial color is red if concentrated and pink if dilute. Further, lowering the pH to about 1 turns it a light yellow color (the pink
disappears). Raising the pH to about 13 makes it go colorless. Both of these changes are reversible. |
It looks like you paid $50 for a +20 year old used bottle of about 28g of something. Ouch!!
Since you have already purchased it why not try it in a Schiff test.
You could try paper chromatography it may tell you if you have a mixture but the three dyes are very similar and ideally you would want genuine
samples of the three dyes for comparison.
Many triarylmethane dyes are ph indicators and have an extra colour change at extreme ph values.
from wiki: phenolphthalein colour change
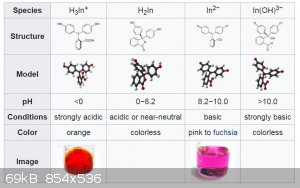
[Edited on 2/16/2020 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by wg48temp9  |
It looks like you paid $50 for a +20 year old used bottle of about 28g of something. Ouch!!
Since you have already purchased it why not try it in a Schiff test.
You could try paper chromatography it may tell you if you have a mixture but the three dyes are very similar and ideally you would want genuine
samples of the three dyes for comparison.
Many triarylmethane dyes are ph indicators and have an extra colour change at extreme ph values.
from wiki: phenolphthalein colour change
[Edited on 2/16/2020 by wg48temp9] |
Thanks for the reply. Yes, I did have to pay quite a bit but I looked around and I didn't see any less expensive easy way for me to get fuchsin.
I will try the Schiff test.
I did try paper chromatography and found no separation, but I was just using strips of paper towel and water (for one test) and acetone (for another).
I'm not sure how effective that combination is at separating these closely-related dyes (like you said).
I am familiar with the extreme pH behavior of phenolphthalein but I am not sure what the expected behavior of rosanaline, pararosanaline, or any of
the other candidates here would be. I did not detect any color change at moderate pH (between 1 and 12) although I didn't do a very systematic test -
just dropwise added HCl to one sample and NaOH soln to another. Nothing happened in either until I had added quite a bit of each.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
I guess I should note that I got 41 grams. The number in front of "Oz." on the original label was not readable. It may have been a 2, although the
bottle was pretty full.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
I am in the process of preparing Schiff's reagent using 0.25g of this dye, 0.25g of sodium bisulfite, 10mL of 1M HCl, and distilled water to make up
about 60mL. The color is still quite red (although I can now see through it whereas I could not when first mixed) after about 3 hours. I will let it
go overnight and see whether it mostly de-colorizes.
I also did an octanol-water partition test. It shows that the dye produces a slightly darker shade in water than in 1-octanol, but that could just be
that there is only one compound and it is more soluble in water than 1-octanol. Hard to tell because even if it is a mixture, it could be that all the
dyes have close to the same shade and wouldn't produce a very different color even if they separated between water and octanol.
First picture is after shaking and allowing to settle for about 1 hour. There is considerable octanol emulsified in the water phase so I then
centrifuged it and the second photo shows a much more subtle difference between the two phases.
 
|
|
|
RadicallyStabilized
Harmless

Posts: 35
Registered: 3-10-2018
Member Is Offline
|
|
You probably need to add more (meta)bisulfite.
I just tested my basic fuchsine (which I bought years ago from a reputable vendor). A spatula of this powder (weight not known, sorry, due to scale
malfunction, but probably less than 100 mg) was dissolved in 10 ml of water, giving a deep red solution. I added about 25 ml of ca. 5% HCl and 475 mg
of dissolved sodium metabisulfite with no color change. More solid metabisulfite was then added with stirring. The color became gradually less red. I
estimate that it required about 1 g of the metabisulfite, if not more, until the color change was complete.
The solution didn't become totally colorless, a slight brown tinge remained and small bits of a brown solid could be seen.
A drop of acetaldehyde instantly turned the solution deep purple. A solution containing piperonal caused a pink color. Adding a little vanillin did
not change the color (this is expected, as aldehydes with aromatic hydroxyls do not react with Schiff's reagent).
Hope this helps, sorry for the sloppy execution. Exact proportions don't seem to be critical to me, though.
Everyday consciousness classifies and subordinates, coerces under patterns of easy manipulation and disregards the essential.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by RadicallyStabilized  | You probably need to add more (meta)bisulfite.
I just tested my basic fuchsine (which I bought years ago from a reputable vendor). A spatula of this powder (weight not known, sorry, due to scale
malfunction, but probably less than 100 mg) was dissolved in 10 ml of water, giving a deep red solution. I added about 25 ml of ca. 5% HCl and 475 mg
of dissolved sodium metabisulfite with no color change. More solid metabisulfite was then added with stirring. The color became gradually less red. I
estimate that it required about 1 g of the metabisulfite, if not more, until the color change was complete.
The solution didn't become totally colorless, a slight brown tinge remained and small bits of a brown solid could be seen.
A drop of acetaldehyde instantly turned the solution deep purple. A solution containing piperonal caused a pink color. Adding a little vanillin did
not change the color (this is expected, as aldehydes with aromatic hydroxyls do not react with Schiff's reagent).
Hope this helps, sorry for the sloppy execution. Exact proportions don't seem to be critical to me, though. |
Thanks for doing this test for me!
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Thanks to everyone for their help. I took RadicallyStabilized's suggestion that I probably needed more reducing agent. In my case, I was using sodium
bisulfite not metabisulfite, but I have seen Schiff's reagent recipes both ways.
Ultimately, I used about 250mg fuchsin, between 1 and 1.5 grams of NaHCO3, about 0.04 moles HCl, and about 100mL distilled water. After mixing until
everything dissolved and it stopped becoming more colorless, I added about 1 gram of activated carbon (which I had seen as a suggestion in one
recipe), stirred that for about 15 minutes, and then filtered it first through a coffee filter and then through a 220nm PES syringe-driven filter. The
result was a very clear, slightly yellow-brown-tinted liquid. I'm pretty sure I had an excess of bisulfite because when poured, this solution released
considerable SO2.
I tested 5mL of it with about 150uL saturated formaldehyde (H2CO) in water and got a very very deep purple color. Just to be sure, I also made a
similar solution with no fuchsin (just HCl, NaHCO3, and water) with approximately the same proportions and put 150uL of formaldehyde in it with no
color change. So I think that confirms that whatever I have, I am able to make a version of Schiff's reagent with it.
I also tested the original sample that I wanted to test for aldehydes (I suspect acrolein) and since I suspected that the concentration is very low, I
added about 0.5mL of it to 5mL of the Schiff reagent and after shaking and letting stand for about 30 seconds, a faint purple color developed.
See the attached image.

|
|
|
RadicallyStabilized
Harmless

Posts: 35
Registered: 3-10-2018
Member Is Offline
|
|
Ok, seems to work, good.
Are you sure you've got the bisulfite NaHSO3? Wikipedia says it's only stable in solution. That's why the metabisulfite (aka disulfite or pyrosulfite,
NaS2O5) is commonly used.
Everyday consciousness classifies and subordinates, coerces under patterns of easy manipulation and disregards the essential.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by RadicallyStabilized  | Ok, seems to work, good.
Are you sure you've got the bisulfite NaHSO3? Wikipedia says it's only stable in solution. That's why the metabisulfite (aka disulfite or pyrosulfite,
NaS2O5) is commonly used. |
Yes, I'm sure. Where does it say that it's only stable in solution? I looked at the wikipedia article and I don't see that.
|
|
|
RadicallyStabilized
Harmless

Posts: 35
Registered: 3-10-2018
Member Is Offline
|
|
It's in the German wikipedia: https://de.wikipedia.org/wiki/Natriumhydrogensulfit
If my German doesn't fail me, "Es ist außerhalb von wässrigen Lösungen nicht stabil" means "it's not stable outside of aqueous solutions"...
Everyday consciousness classifies and subordinates, coerces under patterns of easy manipulation and disregards the essential.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Very interesting. The English and German Wikipedia entries differ. The English one states "Appearance: White Solid" in the infobox. There is a
discussion under the Talk page where someone links to an article stating that it does not exist except in solution, and he says he is going to fix it
if there are no objections, no objections are shown and that was in 2012 and it still says solid.
All I can say is that at least one of the recipes for Schiff's reagent which I found says to use "sodium bisulfite" and I have a bottle labeled
"sodium bisulfite" so I used it. Maybe it is really the metabisulfite. Here is a link to the vendor I got it from, which shows a photo of the bottle:
https://www.amazon.com/Sodium-Bisulfite-Fine-Granular-Powder...
The CAS# and molecular weight on the label match the wikipedia entry.
There are several other vendors on eBay and Amazon which also list chemicals under that name.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
I just noticed that on the talk page of the German wiki there is also a discussion of whether it exists as a solid and someone says it does, and
someone else says that it is usually a mixture of the bisulfite and metabisulfite. I wonder if it is quasi-stable when in the presence of the
metabisulfite and slowly converts to it.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Sigma Aldrich also thinks it exists:
https://www.sigmaaldrich.com/catalog/product/sigald/243973
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
And here is the counter-point:
https://pubs.acs.org/doi/abs/10.1021/ed077p830.1
(Freely-downloadable 1 page article/letter)
|
|
|
RadicallyStabilized
Harmless

Posts: 35
Registered: 3-10-2018
Member Is Offline
|
|
Now this thread has evolved from "fuchsin confusion" to "bisulfite confusion"...
I'm not really sure what to make of this. The English wikipedia is imo technically incorrect when it states that the "salt of bisulfite can be
prepared by bubbling sulfur dioxide in a solution of sodium carbonate in water". In fact this makes only a solution of the salt. But it doesn't say
how to isolate the salt itself which seems to be relevant here.
Pubchem info is contradictory as well, some sources say it's a solid, some it's a liquid.
Maybe you can test the solubility of the salt you have? NaHSO3 is 42 g/100 ml (Wikipedia, I assume at 20 °C); Na2S2O5 (I made a mistake in the
formula above) is 65.3 g/100 ml at 20 °C. That should be enough of a difference to allow identification.
Or maybe Sigma have now found a way to prepare solid NaHSO3... interestingly, at carlroth.com you won't find the sodium bisulfite as a solid. They
only have solutions (search for "bisulphite").
I just checked my old Merck index, it says:
"The bisulfite of commerce consists chiefly of sodium metabisulfite, Na2S2O5, and for all practical purposes possesses the same properties as the true
bisulfite."
Now this seems to be a decisive statement at least.
Everyday consciousness classifies and subordinates, coerces under patterns of easy manipulation and disregards the essential.
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by RadicallyStabilized  | Now this thread has evolved from "fuchsin confusion" to "bisulfite confusion"...
I'm not really sure what to make of this. The English wikipedia is imo technically incorrect when it states that the "salt of bisulfite can be
prepared by bubbling sulfur dioxide in a solution of sodium carbonate in water". In fact this makes only a solution of the salt. But it doesn't say
how to isolate the salt itself which seems to be relevant here.
Pubchem info is contradictory as well, some sources say it's a solid, some it's a liquid.
Maybe you can test the solubility of the salt you have? NaHSO3 is 42 g/100 ml (Wikipedia, I assume at 20 °C); Na2S2O5 (I made a mistake in the
formula above) is 65.3 g/100 ml at 20 °C. That should be enough of a difference to allow identification.
Or maybe Sigma have now found a way to prepare solid NaHSO3... interestingly, at carlroth.com you won't find the sodium bisulfite as a solid. They
only have solutions (search for "bisulphite").
I just checked my old Merck index, it says:
"The bisulfite of commerce consists chiefly of sodium metabisulfite, Na2S2O5, and for all practical purposes possesses the same properties as the true
bisulfite."
Now this seems to be a decisive statement at least. |
OK, I finally had a chance to check the solubility.
For the unknown (called sodium bisulfite on the bottle), I was able to dissolve just under 3.36g in 5mL which corresponds to 67.2g/mL. I say "just
under" because in the end condition there was a tiny amount which did not dissolve.
To check my method, I also tried it with known sodium metabisulfite. There, I was able to dissolve just under 3.59g/5mL or 71.8g/100mL.
Caveats: My sodium metabisulfite is labelled as "technical grade" and is very slightly yellow.
The "sodium bisulfite" doesn't have a grade or purity listed but it is pretty pure white.
Both produced a slightly yellow solution when saturated but the "sodium bisulfite" produced much more gas on dissolving and had a tendency to form a
lot more bubbles and act in a soap-like manner. I did not clean the test tube (plastic screw-top centrifuge vial) before trying the first one (the
unknown) although it looked very clean. I did clean it (with several water rinses) between the two tests. I also accidentally lost a few drops (maybe
0.5mL) of water+some solute during the first test when I opened the tube to vent it, but did not lose any on the second one.
Both solutes seemed to cool the water slightly when they dissolved - especially at lower concentration. I used my hand to warm it back up , so the
temperature was not very well controlled. Air temp is 23C and so would be the distilled water I used.
So, it looks like the "sodium bisulfite" I have is indeed sodium metabisulfite as you claimed.
Thanks for your help!
|
|
|
sbreheny
Hazard to Others
  
Posts: 145
Registered: 30-1-2014
Member Is Offline
Mood: No Mood
|
|
Quick note - I confirmed that the enthalpy of solution of sodium metabisulfite is positive ( +27kJ/mol ), so it should indeed cool somewhat when it
dissolves as I observed by feel.
|
|
|
RadicallyStabilized
Harmless

Posts: 35
Registered: 3-10-2018
Member Is Offline
|
|
Cool that you took the time to investigate this! I wonder where the bubbles/foam comes from though. Probably some kind of impurity. Mine is labeled
"food grade, > 97%" and when I dissolve it there's no bubbles, just a faint smell of SO2.
Everyday consciousness classifies and subordinates, coerces under patterns of easy manipulation and disregards the essential.
|
|
|