DrIronic101
Harmless

Posts: 20
Registered: 16-9-2019
Location: Western United States
Member Is Offline
Mood: Insomniated
|
|
Vanillin demethylation via aluminum tribromide -- still confused.
I'm still confused exactly the reaction mechanism behind this. I'm aware that methyl bromide is formed from the conversion of vanillin's ether into an
alcohol, but beyond that I'm confused as to what all is happening in this reaction. It would make sense to me for h2o to be involved in this reaction,
but I'm assuming that's not the case. Any smarter minds that know what's happening in this rxn would be greatly appreciated, thanks y'all.
"Technically, chemistry is the study of matter, but I prefer to see it as the study of change. Electrons—they change their energy levels. Molecules
change their bonds. Elements—they combine and change into compounds. Well, that’s all of life, right? It’s the constant. It’s the cycle.
It’s solution, dissolution, just over and over and over. It is growth, then decay, then transformation." -Walter White on what chemistry is.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
.
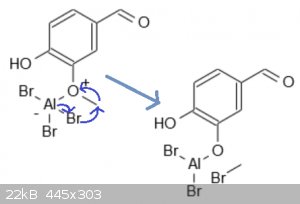
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
DrIronic101
Harmless

Posts: 20
Registered: 16-9-2019
Location: Western United States
Member Is Offline
Mood: Insomniated
|
|
C̶o̶o̶l̶,̶ ̶t̶h̶a̶t̶ ̶a̶c̶t̶u̶a̶l̶l̶y̶ ̶m̶a̶k̶e̶s̶ ̶s̶e̶n̶s̶e̶.̶ ̶D̶i̶d̶n̶'̶t̶ ̶k̶n̶o̶w̶ ̶t̶h̶e̶
̶a̶l̶u̶m̶i̶n̶u̶m̶ ̶a̶t̶t̶a̶c̶h̶e̶d̶ ̶d̶i̶r̶e̶c̶t̶l̶y̶ ̶t̶o̶ ̶t̶h̶e̶ ̶o̶x̶i̶d̶e̶.̶ ̶D̶o̶ ̶y̶o̶u̶
̶k̶n̶o̶w̶ ̶h̶o̶w̶ ̶t̶h̶e̶ ̶a̶l̶u̶m̶i̶n̶u̶m̶ ̶d̶i̶b̶r̶o̶m̶i̶d̶e̶-̶o̶x̶i̶d̶e̶ ̶i̶s̶ ̶t̶h̶e̶n̶
̶r̶e̶d̶u̶c̶e̶d̶ ̶t̶o̶ ̶t̶h̶e̶ ̶a̶l̶c̶o̶h̶o̶l̶?̶ ̶S̶o̶r̶r̶y̶ ̶t̶o̶ ̶a̶s̶k̶,̶ ̶b̶u̶t̶ ̶t̶h̶i̶s̶
̶r̶x̶n̶ ̶h̶a̶s̶ ̶b̶o̶g̶g̶l̶e̶d̶ ̶m̶y̶ ̶m̶i̶n̶d̶ ̶f̶o̶r̶ ̶a̶g̶e̶s̶ ̶a̶n̶d̶ ̶I̶'̶m̶ ̶t̶r̶y̶i̶n̶g̶
̶t̶o̶ ̶f̶i̶g̶u̶r̶e̶ ̶i̶t̶ ̶o̶u̶t̶.̶
Never mind, I went back and read CycloKnight's overview of this rxn. Thanks for that rxn mechanism, I wasn't aware the AlBr3 reacts with the oxide in
that manner.
[Edited on 5-3-2020 by DrIronic101]
"Technically, chemistry is the study of matter, but I prefer to see it as the study of change. Electrons—they change their energy levels. Molecules
change their bonds. Elements—they combine and change into compounds. Well, that’s all of life, right? It’s the constant. It’s the cycle.
It’s solution, dissolution, just over and over and over. It is growth, then decay, then transformation." -Walter White on what chemistry is.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Couldn't this be used to do an one pot synthesis of the methylenedioxide aldehyde? First add an amount of aluminum bromide, then add dibromomethane
and some NaOH.
Does the demethylation run in DCM?
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
@Tsjerk there are a few procedures in the rhodium archive using AlX3 in pyridine. I think they demethylated both vanillin and eugenol.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
The demethylation is easy, the dimethylation is well described as well. An one pot reaction would be interesting.
If the first reaction runs in DCM there wouldn't even be a need for dibromomethane. Just dump vanille in DCM, add some aluminum bromide, reflux, add
NaOH in water, reflux, have your second ring.
[Edited on 5-3-2020 by Tsjerk]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
@Tsjerk sorry I misunderstood what you said. I have a little experience with the demethylation/methylenation with DCM. You may be on to something with
running the reaction in DCM. I think pyridine is needed as a proton sponge to push the reaction forward. What is the purpose of NaOH? To destroy the
AlBr3? I would think the tribromide could be recovered to some extent.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Tsjerk: the rxn conditions are very acidic; aluminum "esters" are not very good nucleophiles. You might be able to do a one-pot if you add
dihalomethane after all the MeBr boils away and basify somehow, but it's not plug-and-chug.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
brubei
Hazard to Others
  
Posts: 187
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tsjerk  | Couldn't this be used to do an one pot synthesis of the methylenedioxide aldehyde? First add an amount of aluminum bromide, then add dibromomethane
and some NaOH.
Does the demethylation run in DCM? |
Quote: Originally posted by njl  | | @Tsjerk there are a few procedures in the rhodium archive using AlX3 in pyridine. I think they demethylated both vanillin and eugenol.
|
the reaction is very tricky, and solvent dependent. It needs acetonitrile and other scavenger to get good
yield and avoid heavy workup.
https://doi.org/10.1002/slct.201803469
I'm French so excuse my language
|
|
|
chemistry007
Harmless

Posts: 19
Registered: 14-6-2019
Member Is Offline
|
|
I tryed once to do it with eugenol....a real mess..i used to generate aluminium iodine insitu with iodine and aluminium powder...
You stay with a unfiltrable cake...really big mess. Off curse, i suppose i may have better result if i try and try...I tryed once and let it down.
Chemplayer did a nice video if i remember, but cant find the video.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
chemistry007: The best method found by CycloKnight uses Al + Br2 in toluene with a slight excess of Br to generate o/p-bromotoluene in situ as a
cosolvent, which improves the solubility of the reactants. This system seems to improve yields significantly.
Tsjerk: According to the famous catechol methylenation paper by Bonthrone and Cornforth, you want a highly dipolar aprotic solvent to encourage the
reaction with DCM and limit its volatility. DMF or DMSO are preferred; propylene carbonate may be a good OTC substitute. But this is rather different
from the demethylation system.
[Edited on 19-3-2020 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
chemistry007
Harmless

Posts: 19
Registered: 14-6-2019
Member Is Offline
|
|
I check in litterature...best yield 99% ( dont really belive, but it's what they say), is AlI3, in MeCN and pyridine...
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The literature is not wrong, but nobody bothers making acetonitrile and pyridine for this rxn. I'd imagine that if you're having trouble making
aluminium iodide, distilling pyridine might be a problem.
The bromotoluene method is the best practical technique we know of.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
chemistry007
Harmless

Posts: 19
Registered: 14-6-2019
Member Is Offline
|
|
The matter was not to making aluminium iodine, but the cacke after that was very messy and i didnt try this methode in litterature, i think it was
some thing that i found from internet. But i think it can be better to work with the bromotoluene, if it works and is not messy...
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
chemistry007: It's very important to read the reports by people who actually do the experiments, because key technical details which may be mentioned
only briefly in papers will be emphasized by posters here (and elsewhere).
For example, in the demethylation of eugenol, using an inert atmosphere is extremely important to obtain a good yield, as CycloKnight's tests showed.
This might be just two words ("under nitrogen") in the experimental details of a paper because chemists in professional labs can easily take such
precautions, but amateurs will avoid difficulty whenever possible and thereby find out exactly what protective measures are most important.
When I first replied to you, I remembered that CycloKnight had found a method that worked, but I didn't remember offhand exactly why. Use the search
engine.
[Edited on 29-3-2020 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|