| Pages:
1
2 |
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Here is a final project plan
It would be fun to discover the smells of the cleaved menthol carboxylic acid, and the ester. Almost all organic compounds have some unique aroma.
I dunno if the fisher esterification step would have some side reaction with the ketone.
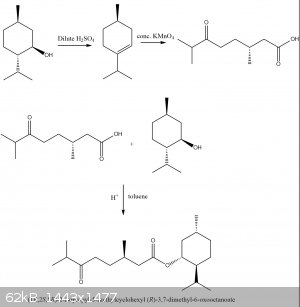
interesting, a derivative of that carboxylic acid is used in flavoring https://www.sigmaaldrich.com/catalog/product/aldrich/w314220...
IF you have access to Scifinder, it has some information on 3,7-dimethyl-6-oxooctanoic acid
some esters of menthol , such as the acetate ester, are used in flavorings and oils. Menthyl acetate is peppermint aroma. https://pubchem.ncbi.nlm.nih.gov/compound/1S_2R_5S_-2-Isopro...
i think fischer esterification will work for menthyl acetate but i found this interesting journal https://pubs.acs.org/doi/10.1021/ed5007037
[Edited on 22-4-2020 by Cou]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Cyclohexanol will react with hot nitric acid to give adipic acid- I wonder if menthol would give one main product or just a mess under the same
conditions.
(The reaction of cyclohexanol with nitric acid is not a reaction for the faint of heart- it is autocatalytic, so it often happens that there's no
reaction, and there's no reaction, and there's suddenly a volcano. I find that adding a drop or two of sodium nitrite solution to the acid before
adding the cyclohexanol prevents this.)
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
Alright! Sorry for the delay, I would have done it last week but there were interruptions which prevented my doing so.
Menthene did not smell very different, however when the permanganate solution was added to it the it smelt more... sweeter? Kind of like peppermint
candy. I tried making menthone with the acidified permanganate, to see what it smelt like and it smelt like peppermint candy, but less powerful. Could
the sulfuric in the KMnO4 solution be dehydrating the menthol faster then the KMnO4 can oxidize it?
This was mainly a small scale test, so my numbers were a little bit messy(so as to spare your eyes I shall withhold them), but I'll do a larger scale
and write down proper lab report(I might film it too), when I am not working on so many projects at once.
Here is a rough overview of what I did.
I used 10% H2SO4 in excess to make the menthene. Not much to say about it except it looked and smelt like menthol.
Added a few drops of conc H2SO4 to some saturated KMnO4 solution, with some menthene, and kept it at menthenes melting point. Took about 15 hours. I
then steam distilled It, and got some immiscible liquid on top of the water.
I have yet to get my hands on some toluene, but I think I'm getting close to finding some.
Thank you everyone for your help!
[Edited on 5-5-2020 by Kobold vor NH4]
"I don't need no excuse for being what I am"
-----Frank Zappa
|
|
|
| Pages:
1
2 |
|