Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
Sorbic acid esters
Hello Friends,
I have been synthesizing sorbic acid ester and will be doing write-ups of them all. So far I have synthesized methyl sorbate, isopropyl sorbate and am
working on n-pentyl sorbate. I have n-butanol and plan on doing that reaction tomorrow. When its easier to purchase at the pharmacy I will be getting
ethanol and synthesizing ethyl sorbate.
Syn
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
What are these useful for? Do they have some specific smell or something?
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
I'm planning to synthesize allyl sorbate, it should have fruity/pineapple scent.
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
Yes, as with most esters they have a specific small, methyl sorbate is kinda minty but different from methyl salicylate.
Awesome, I never realized how rewarding ester synthesis is. Its something you truly can show off to your friend and they can actually enjoy smelling.
|
|
|
mackolol
Hazard to Others
  
Posts: 458
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Oh yes, my family always complaints about awful smell of my chemistry, so I plan on making some good smelling esters for them someday to recompensate
it a little bit haha.
Also perfumery industry must be really cool looking from chemical point of view.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I hope they smell better than the crotonates I've made, which (at best) smell like mushrooms.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
Definitely, so far the smell good. Methyl sorbate is weird, it's a very familiar minty scent, but different from methyl salicylate. Isopropyl sorbate
smells kinda like apples or pears to me and amyl sorbate smell also similar to apples or pears but different from isopropyl sorbate.
It doesn't surprise though, sorbic/parasorbic acid come from the rowan berry which is a close relative to pears and apples. I am guessing there may be
sorbate esters present in apples and pears that give them their smalls.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
How are you purifying them? Boiling points of 200oC don't lend themselves to distillation.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
With amyl sorbate I boiled it up to 200oC since the alcohol has a lower boiling point amyl alcohol is ~138oC, than filtered to
remove any solid components. With methyl and isopropyl sorbate, methanol and isopropanol are very soluble in water and I used an excess of alcohol
since they are super cheap. I did an NaCl(aq) wash to dry, then anhydrous MgSO4.
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
I wanted to do a synthesis tonight but didn't want anything too long. I decided copper sorbate. I used 2g of sorbic acid and 8g of basic copper
corbonate, I should have used less carbonate. It definitely created something, the crystals are very metallic light blue, and extremely hydrophobic.
I will post a photo, it is on my phone.
Edit
In the photo I put a sample of the carbonate I used in a little dish as a comparison of colour. I should have also put a sample of copper
acetylsalicylate
[Edited on 4-5-2020 by Syn the Sizer]
[Edited on 4-5-2020 by Syn the Sizer]

|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Methyl and other light sorbate esters undergo Diels Alder condensations with maleic anhydride to the appropriate ester of
1-carboxy-4-methyl-1,2,3,4-tetrahydrophthalic anhydride. I started a thread sometime back asking if anyone had any ideas about how to de-hydrogenate
this compound to a substituted phthalic acid but no-one responded with any ideas. The free tetrahydro-tricarboxylic acid tends to decarboxylate
readily (if I recall correctly at the 3 position giving 4-methyl-1,2,3,4-tetrahydro phthalic acid). I wondered if the resulting 4-methyl-phthalic
anhydride would nitrate in the 3 position more readily than normal phthalic anhydride  . .
(it might nitrate in the 5 position too, perhaps more easily given the lesser steric hinderance, b****r.)
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
Quote: Originally posted by Boffis  | Methyl and other light sorbate esters undergo Diels Alder condensations with maleic anhydride to the appropriate ester of
1-carboxy-4-methyl-1,2,3,4-tetrahydrophthalic anhydride. I started a thread sometime back asking if anyone had any ideas about how to de-hydrogenate
this compound to a substituted phthalic acid but no-one responded with any ideas. The free tetrahydro-tricarboxylic acid tends to decarboxylate
readily (if I recall correctly at the 3 position giving 4-methyl-1,2,3,4-tetrahydro phthalic acid). I wondered if the resulting 4-methyl-phthalic
anhydride would nitrate in the 3 position more readily than normal phthalic anhydride  . .
(it might nitrate in the 5 position too, perhaps more easily given the lesser steric hinderance, b****r.) |
Interesting, this is still a little above my level of knowledge right now so I couldn't give any helpful input but I hope somebody here is able to
help.
I like to visualize molecules is this what 1-carboxy-4-methyl-1,2,3,4-tetrahydrophthalic anhydride looks like?
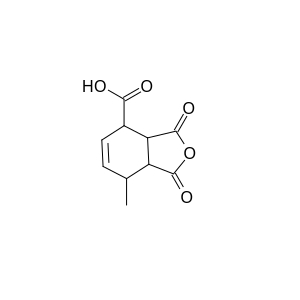
[Edited on 4-5-2020 by Syn the Sizer]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Yes thats right. Do you have access to the SM references forum? If so I have a bundle of papers I collected when I was working on sorbic acid. By the
way sorbic acid undegoes all sorts of reactions to form interesting intermediates; bromine in carbon tetrachloride or DCM converts it into
4,5-dibromo-2-hexenoic acid while refluxing in carbon tetrachloride and excess bromine gives 2,3,4,5-tetrabromohexenoic acid, sodium dithionite
reduces it to 3-hexenoic acid and many other reactions.
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
Quote: Originally posted by Boffis  | | Yes thats right. Do you have access to the SM references forum? If so I have a bundle of papers I collected when I was working on sorbic acid. By the
way sorbic acid undegoes all sorts of reactions to form interesting intermediates; bromine in carbon tetrachloride or DCM converts it into
4,5-dibromo-2-hexenoic acid while refluxing in carbon tetrachloride and excess bromine gives 2,3,4,5-tetrabromohexenoic acid, sodium dithionite
reduces it to 3-hexenoic acid and many other reactions. |
Interesting, I will research these a little more. I do not as of yet have access to the SM reference forum but maybe sometime in the future I will
gain access and will be able to check out your collection.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@ Syn the Sizer; I would be interested in a write-up on the preparation of sorbate esters particularly the lighter end methyl/ethyl sorbates. 
|
|
|
Syn the Sizer
National Hazard
   
Posts: 591
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
Quote: Originally posted by Boffis  | @ Syn the Sizer; I would be interested in a write-up on the preparation of sorbate esters particularly the lighter end methyl/ethyl sorbates.  |
Hey sorry for the late reply, I was honestly just thinking of posting my methyl ester synthesis today. I still haven't done the ethyl ester, the
drugsuor still hasn't replenished their denatured ethanol stock. But I have done methyl, isopropyl, and 1-pentyl, and today am working on 1-butyl. I
will get the methyl ester synthesis wrote up today.
Syn
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Syn the Sizer, looking forward your method. I tried allyl sorbate via allyl alcohol + sorbic acid catalyzed via H3PO4 and H2SO4 (carbonization)
using Dean-Stark trap + hexane for removing reaction water and it is not worth of posting (got only a tar I suppose parasorbic acid + carbon by H2SO4)
an no pleasant scent 
I will very probably try allyl bromide + potassium sorbate - if someone could suggest me suitable solvent I would be very happy 
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Fery, does allyl alcohol act like a dieneophile? Acrolein (=allyl aldehyde) does and therefor undergoes Diels Alder condensation discussed above. You
might want to see if you can prepare sorboyl chloride and condense that with allyl alcohol. Your allyl bromide route should also work.
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Thx Boffis, yes, I should convert sorbic acid into sorbyl chloride.
Here they used thionyl chloride for that:
| Quote: |
Sorbic Acid Chloride.-This compound was produced
by a modification of the method used by Doebner
and Wolff (10). Thionyl chloride (32 Gm.) was
slowly added to 30 Gm. of sorbic acid over a period
of two hours. At the end of three hours, the
reaction between the two compounds had ceased.
The crude product was distilled at 70-80°/25 mm.
The colorless liquid was dried in the presence of
soda lime for four hours and then was redistilled
at 80-84"/20 mm. Consistent yields of 56% were
obtained. |
https://sci-hub.tw/https://onlinelibrary.wiley.com/doi/abs/1...
I'll rather try the K sorbate + allyl bromide route.
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Another method of esterification of sorbic acid is via diazomethane. The latter is developed as a solution in ether and there is no need to isolate
it, usually from "diazold" or N-nitrosomethylurea or one of the similar compounds.
Attachment: MildEsterificationWithDiazomethane.pdf (119kB)
This file has been downloaded 250 times
|
|
|