| Pages:
1
2 |
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
Silica gel mystery?
I have two 1kg bags of silica gel beads sizes about 1mm, but they were bought from some sort of handicraft store so they are not reagent grade.
I need silica gel 60 type, though. How likely it is that this bulk gel is non-usable for this application? I mean, is the type 60 some sort of generic
type?
Sort of stupid question, but I can save almost 100 bucks for not buying a new pack.
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
same boat man.
i bought a bag of silica kitty litter for 5 euros. i started grinding it in a blender and then sieving the powder with a 100 mesh stainless steel
screen. i haven't tested it yet to see how it performs in a chromatography column.
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Chromatography grade silica gel is made under carefully controlled conditions to control the pore size in the particles which is a determinant for
chromatrographic efficiency. Another determinant is particle size. Careful control of these two variables is part of the reason for the expense of
silica gel for chromatography. The OTC silica gel which is mentioned above is just plain ol' silica gel with uncontrolled and perhaps very large pore
sizes. You can grind it and seive it which may give it some utility but I think that you will find that the efficiency and usefulness of separations
will be disappointing. On the other hand, I encourage you to try using what you have and reporting back. You might try separating dyes or inks as your
results could be easily compared to "real" silica gel results which can be found in various sources.
AvB
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by AvBaeyer  | Chromatography grade silica gel is made under carefully controlled conditions to control the pore size in the particles which is a determinant for
chromatrographic efficiency. Another determinant is particle size. Careful control of these two variables is part of the reason for the expense of
silica gel for chromatography. The OTC silica gel which is mentioned above is just plain ol' silica gel with uncontrolled and perhaps very large pore
sizes. You can grind it and seive it which may give it some utility but I think that you will find that the efficiency and usefulness of separations
will be disappointing. On the other hand, I encourage you to try using what you have and reporting back. You might try separating dyes or inks as your
results could be easily compared to "real" silica gel results which can be found in various sources.
AvB |
yup i'm perfectly aware of the cons of using normal silica gel, mine is an experiment, because even if it works ok, i'm going to stick with it. i have
a 50cm column, if i had to fill half of it with chromatografy grade silica gel it would cost me more than the simple compounds i'm tying to purify,
plus i could not buy it anyway as here in italy it is considered a dangerous substance and can't be shipped without the right documentation, that as a
hobbyist, i lack.
i was going to test the silica i has with carrot juice, beta carotenes are very apolar so they should elute pretty easily with hexanes.
plus a question for you, i remember from one of my earlier classes that chromatography silica gel should be activated by boiling it for a few hours in
1M HCl to hydrolyze the siloxy groups to silanol groups and then drying at 110°C to dry the silica, is that right?
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Ubya,
I have NEVER heard of having to boil silica gel in acid to activate it. However, I looked up some basic information about silica gel in Stahl's "Thin
Layer Chromatography" treatise. Indeed during the preparation of chromatographic silica gel the product is sometimes formed by treating certain
starting materials with dilute HCl. There are also analysis procedures which involve the use of HCl in titrations of silica gel. However, nowhere do I
find any mention of HCl treatment required for an sort of post-manufacturing activation. You were mis-informed.
By the way, here in California silica gel is also classified as a hazardous material. Years ago, the company safety office (a real dimwit) wanted
everyone in the research labs to wear hazmat suits when silica gel was being used. He said we needed to be governed by the same rules as a factory
floor based on the msds. I managed to ensure that he did not last long at the company.
By the way, I hope that you are beginning to enjoy a bit more freedom in Italy now that a slow opening up as begun. We had to cancel our spring trip
to Italy and will likely cancel our September trip also. It will likely be the first time in over 20 years that we have not been to Italy. I miss it
dearly.
AvB
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
@AvBaeyer thanks for the correction.
about safety, regulations often go apeshit without really understanding the dangers of a product, silica gel powder = dangerous if inhaled, pretty
much like most of usual lab chemicals...
about the lockdown, yeah in 2 days we are going to be able to go out without autocertification, so it is a big step. the media said that we should be
proud of ourself because we respected the rules, nothing further from the truth. i've been outside 3 times during these 2 months and a half, and i
couldn't belive how stupid people are. masks covering the mouth but not the nose, putting the mask under the chin to speak to the phone, people using
plastic bags or oven waxed paper as makeshift masks (my facepalms were starting to hurt) and in the end the police just checked the city center and
major rich hoods, i live in a poorer hood, and i've never seen controls from the police, so you can go from desert areas to my neighborhood where
everyone is chatting on the streets like nothing is happening.
you did the right thing calceling your trips, in my opinion it's not safe yet.
if you ever visit rome in the future i'll offer you a beer, coffee or a gelato 
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Im interested if the crushed and powdered silica gel works in your column.
Plz report your findings.
If it indeed works you could go to the garden stores and buy a big pack of diatomaceous earth.
Diatomaceous earth is SiO2, but particle and pore size probably isnt same as the SiO2 made for column chromatography.
But i cant imagine garden diatomaceous earth is classed hazardous material, its probably depending on how fine the particles are.
Here in EU one can buy 2.5kg of 60Å, 40-63µm Silica gel for 70Euros on ebay.
That isnt cheap so any research using diatomaceous earth SiO2 or pulverized cat litter would be very interesting.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I'm fairly sure that a lot of the early work on chromatography was done with diatomaceous earth as the stationary phase.
For amateur chemists like us, it's quite likely that "acid washed mud" is good enough.
I can buy it on ebay.
You could, I guess, dissolve it in caustic, filter it and reprecipitate amorphous silica by adding acid; then powder + rinse that product.
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
I would like to try the "Dry Column Vacuum Chromatography" technique that is preformed in a big filtration funnel.
However, i read that Silica Gel 60 (15–40 µm) should be used and i cannot find this 15-40 µm size Silica anywhere.
Maybe i can try some DIY silica that unionised suggested with this technique, who knows it might work.
Here is a link to an description of the "Dry Column Vacuum Chromatography" technique.
https://erowid.org/archive/rhodium/chemistry/equipment/dry-c...
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Diatomaceous earth (aka kieselguhr) has indeed been widely used in the past for chromatography and still finds use in some special situations. The
commercial material that is OTC available is needs substantial purification before it can be effectively used. The inorganic portion is typically
about 90% silica and contains a number of other metal oxides which are may or may not removed. Loss on ignition of crude diatomaceous earth runs
around 4% indicative of organic material and water. It is especially important to remove any organic material. There are references to procedures for
purification of the material for chromatographic use. I do not have those handy at the moment but they should be easy to find if you are interested.
AvB
[Edited on 25-5-2020 by AvBaeyer]
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by AvBaeyer  | Diatomaceous earth (aka kieselguhr) has indeed been widely used in the past for chromatography and still finds use in some special situations. The
commercial material that is OTC available is needs substantial purification before it can be effectively used. The inorganic portion is typically
about 90% silica and contains a number of other metal oxides which are may or may not removed. Loss on ignition of crude diatomaceous earth runs
around 4% indicative of organic material and water. It is especially important to remove any organic material. There are references to procedures for
purification of the material for chromatographic use. I do not have those handy at the moment but they should be easy to find if you are interested.
AvB
[Edited on 25-5-2020 by AvBaeyer] |
i found this process:
mix the diatomaceous earth with Na2CO3, heat to 900-1000°C for a few hours, then let it cool and acid wash it. run solven through it until the
effluent is neutral.
might work on industrial scale but not in a diy setting.
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Update
i finally tested my silica gel powder.
i took a piece of lettuce leaf, i ground it in a mortar with some calcium chloride to absorb any water, and then i added a few ml of acetone.
i ground the mixture for 5 minutes, until the acetone got a drep green color. with a pipette i then took the acetone extract being careful to not pick
any of the plant matter (the calcium chloride/plant matter forms like an immiscible phase with the acetone so it's not hard to separate, no need for
filtration) and i evaporated it to dryness using a jet of air from a pipette and an air pump.
i added 0.5ml of hexanes to the dry extract and i used a few drops of thus solution in the getto chromatography column.
i did a shit job at packing it, thus was my second time i ever packed one (chemistry degree but no lab hours, sad).
i started eluting with 100% hexanes, using the bulb of the pipette to create a positive pressure (too densely packed or 100 mesh is too fine for a
gravity feed).
carotenoids eluted first being the most apolar, i then added a 50/50 mixture of acetone and hexanes to elute the chlorophylls (this was just a quick
test, i didn't prepare any gradient solutions).
for a price of 5 euros for 2.5kg of silica kitty litter, and 30 minutes of blending and sieving this is not bad.
it may not be research lab grade, but to separate a few compounds in an amateur settung i think it is more than enough.
 
 
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
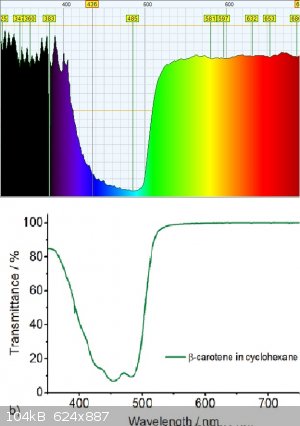
using my little diy crappy VIS-NIR spectrophotometer i took spectrum of the carotenoids, and it matches the transmittance spectrum quite well!
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
The catalyst requires mildly acidic anhydrous conditions which is done in acetone with silica gel.
For catalyst use, should the bulk silica gel work as well as silica gel 60 or do they have differences?
Should the gel be dried before?
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by Refinery  | The catalyst requires mildly acidic anhydrous conditions which is done in acetone with silica gel.
For catalyst use, should the bulk silica gel work as well as silica gel 60 or do they have differences?
Should the gel be dried before? |
grind your silica beads and you are good.
should the silica be dried? i guess so, you need anhydrous conditions right?
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Bmoore55
Hazard to Self
 
Posts: 85
Registered: 23-7-2018
Location: Texas
Member Is Offline
|
|
This is fantastic! I have been wanting to try this for some time now. It's too bad Ubya that you're not in the US, I could have tested your end
product for you. Still a very nice work up.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
BTW Ullmann's suggests that silica gel made as it was 100 years ago (no special processing) has a pore size of 21A. It speaks of 135-185A size, but
nothing in between. It's just weird that half the world knows how to make this 60A size, yet details are nowhere to be found.
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by Bmoore55  | | This is fantastic! I have been wanting to try this for some time now. It's too bad Ubya that you're not in the US, I could have tested your end
product for you. Still a very nice work up. |
Rip, i'll be forever too far from everyone 
@S.C.Wack The information must be out there somewhere, the hard part is finding the right path
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
What is the proper procedure to dry silica gel?
https://www.researchgate.net/post/How_to_extract_water_from_...
This source cites that it degrades faster if dried at high temperatures and recommends using slow drying at 80C.
I was planning on drying it once, and then storing it under acetone because this solvent is used in the catalytic process so it should last as long as
possible.
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
i dried it at 180°C because my toaster over has its max temperature at 180°C(i don't have a PID controller yet)
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
https://www.sciencedirect.com/topics/neuroscience/desiccants
This source cites that: "The moisture in silica gel can be removed by drying at a temperature greater than 110°C. The current USP Chapter <671>
recommends pre-drying silica gel desiccant at approximately 150°C to ensure the complete removal of adsorbed water."
I don't know if its true that heating too much cause degradation?
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Congrats, Ubiya! Your work (the yellow and green liquid in those vials) is very convincing!
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
thanks! i still need to test it a lot 
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Ubya,
Really a nice piece of work! It shows what can be done when experiments are really done and done well. I was a sceptic but you have shown otherwise.
Congratulations.
AvB
|
|
|
Housane
Hazard to Others
  
Posts: 127
Registered: 3-9-2018
Location: Worcester, England
Member Is Offline
Mood: Let’s make
|
|
Ubya, amazing work, what glassware is in those pictures, i really want to try this as i have a load of desicant?
Green QD's so far
Feel free to correct grammar or incorect knknowledge. We are all learning.
|
|
|
| Pages:
1
2 |