| Pages:
1
2
3 |
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
Ok, the solution is back to its original, slightly green coloar, after adding a few drops of hydrogen peroxide it became yellow again. Maybe some
unstable peroxo complex of germanium is formed?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Never heard of one. That type of complex usually requires dangling d or f electrons (see transition metals and rare earths).
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
Well, so I have no idea what is causing that yellow color...
Could it be Ge4+? But why does the color disappear?
|
|
|
thethule
Harmless

Posts: 9
Registered: 23-4-2011
Member Is Offline
Mood: No Mood
|
|
That seems like a very expensive way of getting some Ge. Would it even be pure ge? Even more expensive than getting it from element sales websites.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Maybe I missed it in 3 pages but I am unsure what compound of Ge Mixell is going for. Back in my youth with no internet and no information (or very
little) on Ge chemistry I was trying to alloy Ge. OK, so I thought it would act like a metal, and if I could liquefy it I was going to melt other
metals into it. Heating a 50 gm chunk of pure Ge in a ceramic boat (no flame touching Ge), it began burning in air. Much like a Mg fire I might add.
Clouds of smoke later I had a powder with both yellow/orange and white appearance. Reading about toxicity of at least one Ge - Oxygen compound I left
the room, open for a half day. Still alive all these years later. Anyway, was wondering if this carried out in a fume hood to give an oxide of Ge
would not be a simple, fast starting point to then further react the powder to form other salts of Ge? Much of the Ge went out the door as oxide smoke
so possibly routing this smoke through another vessel with the reactants needed for some other transition might also be a viable procedure?
The Jpeg's below show the only information I possessed back then about Ge chemistry, might still be of use here.
 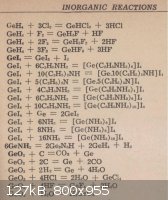
[Edited on 4-25-2011 by IrC]
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
| Pages:
1
2
3 |